Early and locally advanced non-small-cell lung cancer: an update of the ESMO Clinical Practice Guidelines focusing on diagnosis, staging and systemic and local therapy
Published: 01 September 2021
Authors: J. Remon, J-C. Soria and S. Peters, on behalf of the ESMO Guidelines Committee
Clinical Practice Guideline
This update refers to Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up
Ann Oncol. 2017;28(4):iv1–iv21
P. E. Postmus, K. M. Kerr, M. Oudkerk, et al.
Pathology/molecular biology
Diagnosis
The original Table 1 is updated.
Table 1. Work-up for diagnosis and staging
|
Mandatory |
Optional |
|---|---|---|
|
General |
Medical historya |
|
Physical examinationa |
|
|
Assessing comorbidity |
|
|
PS |
|
|
|
Imaging |
CT thorax and upper abdomena |
X-ray thoraxb |
PET-CTa |
Bone scintigraphy |
|
MRI brainc |
Contrast-enhanced CT brain |
|
|
Laboratory |
Blood cell counts |
|
Renal function |
|
|
Liver enzymes |
|
|
|
Bone parametersd |
|
|
Cardiopulmonary function |
FVC, FEV1, DLCO |
|
ECG |
|
|
If indicated: CPET |
Ejection fraction, CAG |
|
|
Tissue procurement |
Bronchoscopyc,e |
|
EBUS/EUS mediastinal nodesa |
Mediastinoscopy |
|
CT-guided biopsy |
|
|
Genomic profiling |
EGFR mutation status |
ALK fusion status |
Other biomarkers |
PD-L1 expression (for unresectable NSCLC) |
PD-L1 expression (for completely resected NSCLC) |
CAG, coronary angiography; CPET, cardiopulmonary exercise testing; CT, computed tomography; DLCO, diffusing capacity of the lungs for carbon monoxide; EBUS, endoscopic bronchial ultrasound; ECG, electrocardiogram; EUS, endoscopic ultrasound; FEV1, forced expiratory volume in 1 second; FVC, forced expiratory vital capacity; MRI, magnetic resonance imaging; NSCLC, non-small-cell lung cancer; PD-L1, programmed death-ligand 1; PET-CT, positron emission tomography computed tomography; PS, performance status.
a Tests needed for clinical staging.
b X-ray could be used as a first test in case of suspicious of lung cancer. However, a CT scan is recommended for those patients with risk factors or high clinical suspicious and a negative X-ray.
c See text.
d Optional, at physician’s discretion upon suspicion. Bone scan or PET-CT to be performed in case of suspicion for bone metastases.
e Depending on site and size of tumour with biopsy/aspiration/brush/washing.
Staging and risk assessment
Locoregional staging
The original Figure 1 is updated.
Figure 1. Suggested algorithm for locoregional lymph node staging in patients with non-metastatic NSCLC.
Red: surgery; turquoise: combination of treatments or other systemic treatments; white: other aspects of management.
CT, computed tomography; EBUS, endoscopic bronchial ultrasound; EUS, endoscopic ultrasound; FDG, fluorodeoxyglucose; LN, lymph node; NPV, negative predictive value; NSCLC, non-small-cell lung cancer; PET, positron emission tomography; VAM, video-assisted mediastinoscopy.
a In tumours ˃3 cm (mainly in adenocarcinoma with high FDG uptake), invasive staging should be considered.
b Depending on local expertise to adhere to minimal requirements for staging.
c Endoscopic techniques are minimally invasive and are the first choice if local expertise with EBUS/EUS needle aspiration is available.
d Due to its higher NPV, in case of PET-positive or CT-detected mediastinal LN enlargement, negative endoscopic staging (EBUS or EUS) and high clinical suspicion with availability of VAM, nodal dissection or biopsy is recommended.
Adapted with permission from De Leyn et al.2
Treatment of early stages (stages I-IIIA)
The original section ‘Treatment of early stages (Stages I-II)’ is renamed ‘Treatment of early stages (Stages I-IIIA)’. The original recommendations are updated and a new treatment algorithm provided (Figure 6). The ESMO-MCBS table is updated to include osimertinib (Table 6).
Systemic therapy
Adjuvant treatment with targeted therapies
Adjuvant osimertinib for resected IB-IIIA NSCLC with EGFR exon 19 deletions or exon 21 L858R mutations. Osimertinib is approved by both the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) as adjuvant therapy after complete tumour resection in patients with stage IB-IIIA NSCLC whose tumours have epidermal growth factor receptor (EGFR) exon 19 deletions or exon 21 L858R mutations [I, A; European Society for Medical Oncology-Magnitude of Clinical Benefit Scale (ESMO-MCBS) v1.1 score: A].
Efficacy was demonstrated in a randomised, double-blind, placebo-controlled trial (ADAURA, NCT02511106) in patients with centrally confirmed EGFR exon 19 deletions or exon 21 L858R mutation-positive stage IB-IIIA NSCLC who had complete tumour resection, with or without prior adjuvant chemotherapy (ChT).3,4 A total of 682 patients were randomised (1:1) to receive three years of osimertinib 80 mg orally once daily or placebo following recovery from surgery and standard adjuvant ChT, if given. The major efficacy outcome measure was disease-free survival (DFS) in patients with stage II–IIIA NSCLC determined by investigator assessment. With a median follow-up for the primary endpoint of 22.1 months in the osimertinib arm and 14.9 months in the placebo arm, the median DFS was not reached [(38.8 months-not evaluable (NE)] in patients on the osimertinib arm compared with 19.6 months (16.6-24.5 months) on the placebo arm [hazard ratio (HR) 0.17; 99.1% confidence interval (CI) 0.11-0.26; P < 0.0001]. DFS in the overall study population was a secondary efficacy outcome measure; the median was not reached (NE-NE) in patients on the osimertinib arm compared with 27.5 months (22.0-35.0 months) on the placebo arm (HR 0.20; 99.1% CI 0.14-0.30; P < 0.0001).3,4 The DFS benefit with osimertinib occurred in patients who received adjuvant ChT (HR 0.16, 95% CI 0.10-0.26), and among those who did not (HR 0.23, 95% CI 0.13-0.40), knowing that ChT administration was not a stratification factor and this decision was left to the investigator.3
The recommended osimertinib dose for adjuvant treatment of early-stage NSCLC is 80 mg orally once daily, with or without food, until disease recurrence, or unacceptable toxicity, or for up to 3 years.
Neoadjuvant or adjuvant immune checkpoint inhibitors
Unpublished data from phase III clinical trials have reported that in patients with stage IB-IIIA NSCLC, neoadjuvant immune checkpoint inhibitors (ICIs) plus ChT increases the rate of pathological complete response compared with ChT. Likewise, in the same setting, adjuvant ICI with anti-PD-L1 leads to increased DFS versus best supportive care (BSC) for patients with PD-L1-positive tumours.
The recent phase III IMpower-010 trial assessed the role of adjuvant atezolizumab 1200 mg every 3 weeks for 16 cycles versus BSC in completely resected stage IB-IIIA NSCLC patients following recovery from surgery and standard adjuvant cisplatin-based ChT.5 In hierarchical testing, atezolizumab significantly improved the DFS versus BSC in PD-L1-expressing tumour cells ≥1% (SP263 assay) stage II-IIIA NSCLC (median DFS not reached vs 35.3 months, HR 0.66 [95% CI 0.50-0.88], P = 0.0039), as well as in all randomised stage II-IIIA tumours (median DFS 42.3 vs 35.3 months; HR 0.79 [95% CI 0.64-0.96], P = 0.0205). Finally, in the intention-to-treat population, DFS did not cross significance boundary [HR 0.81 (95% CI 0.67-0.99), P = 0.04].5 However, atezolizumab does not have EMA approval in this setting yet.
The primary analysis population from the three-arm CheckMate 816 trial reported that nivolumab plus platinum-based ChT for 3 cycles as neoadjuvant strategy in patients with stage IB-IIIA NSCLC significantly improved the primary endpoint of pathological complete responses compared with ChT alone [24.0% versus 2.2%, odds ratio (OR) 13.94, 99% CI 3.49-55.75, P < 0.0001]. This benefit was consistent across disease stages, histologies, tumour mutational burden and PD-L1 expression levels.6 However, there are no data yet on outcome with this strategy from this phase III trial. Therefore, in stage IB-IIIA NSCLC, the immune strategy in the (neo)adjuvant setting using ICIs ± CT is not yet standard. Several large phase III clinical trials are still ongoing with immune strategy in the (neo)adjuvant stage IB-IIIA clinical scenario.
Postoperative radiotherapy
The original recommendations are updated.
Recommendations
Adjuvant ChT
- Adjuvant ChT should be offered to patients with resected tumour–node–metastasis (TNM) 8th edition stage IIB and III NSCLC [I, A] and can be considered in patients with T2bN0, stage IIA resected primary tumour >4 cm [II, B]. Pre-existing comorbidity, time from surgery and postoperative recovery need to be taken into account in this decision taken in a multidisciplinary tumour board [V, A].
- For adjuvant ChT, a two-drug combination with cisplatin is preferable [I, A]. In randomised studies, the attempted cumulative cisplatin dose was up to 300 mg/m2, delivered in three to four cycles.
- When cisplatin administration is not feasible, carboplatin is an accepted alternative [IV, B].
- Although the most frequently studied regimen is cisplatin-vinorelbine, other combinations such as cisplatin and gemcitabine, or docetaxel or pemetrexed (only in adenocarcinoma tumours) could be also feasible [II, B].
- Carboplatin and paclitaxel is a potential ChT option for T2bN0, stage IIA resected primary tumour >4 cm [IV, B].
Adjuvant treatment with targeted therapies
- Osimertinib is indicated for the adjuvant treatment after complete tumour resection in adult patients with stage IB-IIIA NSCLC whose tumours have EGFR exon 19 deletions or exon 21 L858R substitution mutations [I, A].
Postoperative radiotherapy
- Postoperative radiotherapy (PORT) in completely resected early-stage I-IIIA NSCLC is not recommended [I, E].
- In case of microscopic residual tumour (R1) resection (positive resection margin, chest wall), PORT should be considered [IV, B].
- Even if such patients were not included in randomised, clinical trials (RCTs), adjuvant ChT should be considered in patients with R1 resection of stage IIA-IIB-III disease [V, A].
- In case both ChT and radiotherapy (RT) are administered post-R1 surgery, RT may be administered before ChT [V, C].
Treatment of locally advanced stage (stage III)
The original recommendations are updated and a new treatment algorithm provided (see Figure 6). The ESMO-MCBS table is updated for durvalumab (see Table 6).
Resectable LA-NSCLC
PORT after resected stage III NSCLC. PORT should not be used in patients with NSCLC following complete R0 resection and after (neo)adjuvant ChT, as no statistically significant difference in 3-year DFS was shown in a randomised controlled trial.
The large randomised controlled LungART trial presented at ESMO 2020 explored the role of modern mediastinal PORT in patients with completely resected NSCLC with histo/cytologically proven N2 nodal involvement. The study randomised 501 patients with completely resected stage IIIA N2 NSCLC to PORT (54 Gy in 27-30 fractions or no PORT). Safety analysis was carried out in 487 patients.7 LungART is the only large, adequately-powered, high-quality, randomised trial in the modern era to be completed in these patients. The trial selected a well-defined, high-risk patient population (52% of patients had ≥2 N2 nodal stations involved), 91% of whom had undergone positron emission tomography (PET) scans, and 95% of whom had undergone systemic ChT. The quality of surgery performed was clearly specified and also reviewed centrally by a surgical committee.
At a median follow-up of 4.8 years, the DFS HR reported in the trial was 0.85 (95% CI 0.67-1.07; P = 0.16), with median DFS times of 30.5 months for PORT and 22.8 months without PORT. Three-year DFS rates were 47.1% and 43.8% with and without PORT, respectively. There was also no evidence that PORT improved overall survival (OS), with 3-year OS rates of 66.5% and 68.5% with and without PORT, respectively. Not only did PORT fail to provide a survival benefit, but it may actually be harmful for patients, with an incidence of late grade 3-4 cardiopulmonary toxicity twice that in patients without PORT (10.8% versus 4.9%).
Consequently, PORT cannot be recommended for patients with completely resected stage I-III N2 NSCLC. Its potential utility for locoregional disease control in resected stage III N2 disease (decrease of the rate of mediastinal relapse by 50%) is offset by the risk of over-added cardiopulmonary toxicity. Further analysis is needed to determine if certain patients, in particular, could benefit. For patients with incompletely resected R1 stage III NSCLC, post-operative radiotherapy should be considered, with thorough assessment in a multidisciplinary tumour board.
Unresectable LA-NSCLC
The phase III PACIFIC trial randomised (2:1) 713 patients with unresectable, locally-advanced NSCLC without disease progression within the first 42 days after concurrent chemoradiotherapy, to consolidative durvalumab for 1 year or placebo.8 After a median follow-up of 34.2 months, the median OS for durvalumab was reached (47.5 months versus 29.1 months for placebo, HR 0.72, 95% CI 0.59-0.89), and the estimated 5-year OS rates were 42.9% versus 33.4% for durvalumab versus placebo, respectively. The median PFS was 16.9 months for durvalumab and 5.6 months for placebo (HR 0.55, 95% CI 0.45-0.68) with a 5-year PFS rate of 33.1% versus 19.0%, respectively.8
A post-hoc exploratory analysis of the mature survival data requested by licensing European authorities observed that the benefit with durvalumab was not evident in patients with PD-L1 expression <1%. The significance of this observation is disputed.9
Recommendations
PORT after resected stage III NSCLC
- PORT is not beneficial for patients with completely resected stage III N2 NSCLC [I, E] and should only be considered in the setting of residual microscopic or macroscopic disease [IV, B].
Unresectable LA-NSCLC
- The consolidation administration of the ICI durvalumab within 1-42 days after the end of concurrent chemoradiotherapy has demonstrated a survival benefit in unresectable stage III NSCLC and is recommended in patients whose disease has not progressed following platinum-based chemoradiotherapy [I, A] in the intention-to-treat population across all PD-L1 categories and in patients whose tumours express PD-L1 on tumour cells (as per the EMA-approved indication) [I, A; ESMO-MCBS v1.1 score: 4].
Follow-up, long-term implications and survivorship
The original recommendations are updated.
Recommendations
- NSCLC patients treated with radical intent should be followed for treatment-related complications, detection of treatable relapse or occurrence of second primary lung cancer. MDT assessment is required for feasibility check for treatment of locoregional relapse. [III, A].
- Surveillance every 6 months for 2 years with a visit including history, physical examination and contrast-enhanced volume chest and abdominal CT scan at least at 12 and 24 months is recommended, with optional FDG-PET if required, and thereafter an annual visit including history, physical examination and chest/upper abdominal CT scan in order to detect second primary tumours [III, B].
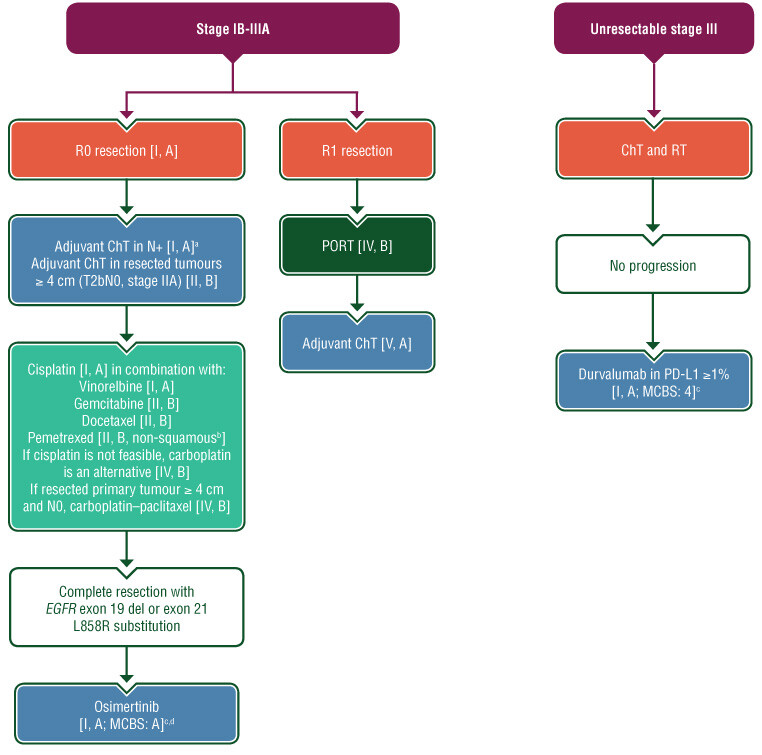
Figure 6: Systemic treatment algorithm for early stage (stage IB-IIIA) and unresectable locally advanced (stage III) NSCLC.
For resection criterion, check Figure 2. Purple: general categories or stratification (symptom); red: surgery; dark green: radiotherapy; blue: systemic anticancer therapy; turquoise: combination of treatments or other systemic treatments; white: other aspects of management.
ChT, chemotherapy; ESMO, European Society for Medical Oncology; MCBS, Magnitude of Clinical Benefit Scale; N+, node-positive; PD-L1, programmed death-ligand 1; PORT, postoperative radiotherapy; RT, radiotherapy.
a For stage IB, adjuvant ChT in primary tumours ≥4 cm [II, B].
b Only in adenocarcinoma tumours.
c ESMO-MCBS v1.1 score for new therapy/indication approved by the EMA since January 1, 2016 and the FDA since January 1, 2020. The score has been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee.
d Primary endpoint of ADAURA trial was DFS in stage II-IIIA according to 7th TNM (T> 5 cm or N+). Adjuvant osimertinib in stage IB (3 cm < T ≤ 5 cm) was a secondary endpoint. Stage was a stratification factor. Therefore, indication for osimertinib in T ≤ 5 cm N0 will follow local recommendations/physician’s discretion [I, B].
Table 6: ESMO-MCBS table for new therapies/indications in early and locally advanced NSCLC.
Therapy |
Durvalumab |
|---|---|
Disease setting |
Consolidation therapy in patients with stage III NSCLC who did not have disease progression after ≥2 cycles of platinum-based CRT |
Trial |
A study of durvalumab as consolidation therapy in patients with locally advanced, unresectable NSCLC (stage III) who have not progressed following definitive, platinum-based, concurrent chemoradiation therapy (PACIFIC)10-14 Phase III NCT02125461 |
Control (median) |
Placebo PFS ITT across all PD-L1 categories control: 5.6 months OS control: 29.1 months 2-year OS control: 55.3% 4-year OS control: 36.3% |
Absolute survival gain |
PFS ITT across all PD-L1 categories gain: 11.6 months OS gain: 18.4 months 2-year OS gain: 11% 4-year OS gain: 13.3% |
HR (95% Cl) |
PFS HR: 0.55 (0.44-0.67) |
QoL/toxicity |
No benefit observed |
ESMO-MCBS scorea |
4b (Form 2a) |
Therapy |
Osimertinib – 3 years |
|---|---|
Disease setting |
Adjuvant treatment after tumour resection EGFR exon 19 deletions or exon 21 (L858R) mutation |
Trial |
A study of osimertinib versus placebo in patients with EGFR mutation positive stage IB-IIIA NSCLC following complete tumour resection with or without adjuvant chemotherapy (ADAURA)15 Phase III NCT02511106 |
Control (median) |
Placebo 2-year DFS control: 52%d |
Absolute survival gain |
2-year DFS gain: 37%d |
HR (95% Cl) |
DFS HR: 0.20 (0.14-0.30)c, d |
QoL/toxicity |
|
ESMO-MCBS scorea |
Ad (Form 1) |
CI, confidence interval; ChT, chemotherapy; CRT, chemoradiotherapy; DFS, disease-free survival; EGFR, epidermal growth factor receptor; EMA, European Medicines Agency; ESMO-MCBS, European Society for Medical Oncology-Magnitude of Clinical Benefit Scale; FDA, Food and Drug Administration; HR, hazard ratio; ITT, intention to treat; NSCLC, non-small-cell lung cancer; OS, overall survival; PD-L1, programmed death-ligand 1; PFS, progression-free survival; QoL, quality of life.
a ESMO-MCBS version 1.1.16 The scores have been calculated by the ESMO-MCBS Working Group and validated by the ESMO Guidelines Committee (https://www.esmo.org/guidelines/esmo-mcbs/scale-evaluation-forms-v1.0-v1.1/scale-evaluation-forms-v1.1).
b EMA approval is limited to PD-L1>1%, based on post-hoc subgroup analysis indicating lack of benefit for patients with PD-L1 <1%,13 FDA approval is based on ITT and is irrespective of PD-L1.
c HR with 99.12% CI.
d Approval was based on all patient data (including stage IB), which was a secondary outcome (statistically significant after hierarchical testing).
Acknowledgements
The ESMO Guidelines Committee acknowledges and thanks the following people who have acted as reviewers for this update: Rolf Stahel (ESMO Guidelines Steering Committee) and Marina Garassino (ESMO Faculty, metastatic NSCLC). George Pentheroudakis (Chief Medical Officer of ESMO) provided coordination and writing support. Catherine Evans and Jennifer Lamarre (ESMO Staff) provided editing support. Nathan Cherny, Chair of the ESMO-MCBS Working Group, Urani Dafni ESMO-MCBS Working Group Member/Frontier Science Foundation Hellas and Giota Zygoura of Frontier Science Foundation Hellas provided review and validation of the ESMO-MCBS scores. Nicola Latino (ESMO Scientific Affairs staff) provided coordination and support of the ESMO-MCBS scores and Angela Corstorphine of Kstorfin Medical Communications Ltd. provided medical writing and editing support in the preparation of the ESMO-MCBS table; this support was funded by ESMO.
Funding
No external funding has been received for the preparation of these guidelines. Production costs have been covered by ESMO from central funds.
Disclosure
JCS reports that he is on the Board of directors for Hookipa Pharmaceuticals, a former full-time employee of AstraZeneca from September 2017 to December 2019 and has shares in Relay Therapeutics and Gritstone Bio. JR reports receipt of honoraria to institute for consultancy, advisory boards and/or lectures from AstraZeneca, Bristol Myers Squibb (BMS), Boehringer Ingelheim, MSD, OSE-Immunotherapeutics, Pfizer, Roche. SP reports consultation/advisory roles for AbbVie, Amgen, AstraZeneca, Bayer, BeiGene, Biocartis, BioInvent, Blueprint Medicines, Boehringer Ingelheim, BMS, Clovis, Daiichi Sankyo, Debiopharm, Eli Lilly, Elsevier, F. Hoffmann-La Roche/Genentech, Foundation Medicine, Illumina, Incyte, IQVIA, Janssen, Medscape, MSD, Merck Serono, Merrimack, Mirati, Novartis, PharmaMar, Phosplatin Therapeutics, Pfizer, Regeneron, Sanofi, Seattle Genetics, Takeda, Vaccibody; Talk in a company’s organised public event: AstraZeneca, Boehringer Ingelheim, BMS, e-cancer, Eli Lilly, F. Hoffmann-La Roche/Genentech, Illumina, Medscape, MSD, Novartis, PER, Pfizer, Prime, RTP, Sanofi, Takeda; Receipt of grants/research support as (sub)investigator in trials (institutional financial support for clinical trials) sponsored by Amgen, AstraZeneca, Biodesix, Boehringer Ingelheim, BMS, Clovis, F. Hoffmann-La Roche/Genentech, GSK, Illumina, Lilly, MSD, Merck Serono, Mirati, Novartis, and Pfizer, Phosplatin Therapeutics.
References
- Postmus PE, Kerr KM, Oudkerk M et al. Early and locally advanced non-small-cell lung cancer (NSCLC): ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2017; 28 (suppl_4): iv1-iv21.
- De Leyn P, Dooms C, Kuzdzal J et al. Revised ESTS guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur J Cardiothorac Surg 2014; 45 (5): 787-798.
- Wu YL, Tsuboi M, He J et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020; 383 (18): 1711-1723.
- Herbst RS, Tsuboi M, John T et al. Osimertinib as adjuvant therapy in patients (pts) with stage IB–IIIA EGFR mutation positive (EGFRm) NSCLC after complete tumor resection: ADAURA. Journal of Clinical Oncology 2020; 38 (18_suppl): LBA5-LBA5.
- Wakelee HA. IMpower010: Primary results of a phase III global study of atezolizumab versus best supportive care after adjuvant chemotherapy in resected stage IB-IIIA non-small cell lung cancer (NSCLC). J Clin Oncol 2021; 39.
- Forde PM, Spicer J, Lu S et al. Nivolumab (NIVO) + platinum-doublet chemotherapy (chemo) vs chemo as neoadjuvant treatment (tx) for resectable (IB-IIIA) non-small cell lung cancer (NSCLC) in the phase 3 CheckMate 816 trial [abstract]. In: Proceedings of the 112th Annual Meeting of the American Association for Cancer Research; 2021 April 10-15. Philadelphia (PA): AACR; 2021. Abstract nr CT003.
- Le Pechoux C, Poural N, Barlesi F et al. LBA3_PR An international randomized trial, comparing post-operative conformal radiotherapy (PORT) to no PORT, in patients with completely resected non-small cell lung cancer (NSCLC) and mediastinal N2 involvement: Primary end-point analysis of LungART (IFCT-0503, UK NCRI, SAKK) NCT00410683. Annals of Oncology 2020; Volume 31, S1178.
- Spigel DR, Faivre-Finn C, Gray JE et al. Five-year survival outcomes with durvalumab after chemoradiotherapy in unresectable stage III NSCLC: An update from the PACIFIC trial. Journal of Clinical Oncology 2021; 39 (15_suppl): 8511-8511.
- Peters S, Dafni U, Boyer M et al. Position of a panel of international lung cancer experts on the approval decision for use of durvalumab in stage III non-small-cell lung cancer (NSCLC) by the Committee for Medicinal Products for Human Use (CHMP). Ann Oncol 2019; 30 (2): 161-165.
- Antonia SJ, Villegas A, Daniel D et al. Durvalumab after Chemoradiotherapy in Stage III Non-Small-Cell Lung Cancer. N Engl J Med 2017; 377 (20): 1919-1929.
- Antonia SJ, Villegas A, Daniel D et al. Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC. N Engl J Med 2018; 379 (24): 2342-2350.
- Hui R, Ozguroglu M, Villegas A et al. Patient-reported outcomes with durvalumab after chemoradiotherapy in stage III, unresectable non-small-cell lung cancer (PACIFIC): a randomised, controlled, phase 3 study. Lancet Oncol 2019; 20 (12): 1670-1680.
- Gray JE, Villegas A, Daniel D et al. Three-Year Overall Survival with Durvalumab after Chemoradiotherapy in Stage III NSCLC-Update from PACIFIC. J Thorac Oncol 2020; 15 (2): 288-293.
- Faivre-Finn C, Vicente D, Kurata T et al. Four-Year Survival With Durvalumab After Chemoradiotherapy in Stage III NSCLC-an Update From the PACIFIC Trial. J Thorac Oncol 2021; 16 (5): 860-867.
- Wu YL, Tsuboi M, He J et al. Osimertinib in Resected EGFR-Mutated Non-Small-Cell Lung Cancer. N Engl J Med 2020; 383 (18): 1711-1723.
- Cherny NI, Dafni U, Bogaerts J et al. ESMO-Magnitude of Clinical Benefit Scale version 1.1. Ann Oncol 2017; 28 (10): 2340-2366.