Patients with inoperable locally recurrent or metastatic, treatment-naïve, squamous cell carcinoma of the anal canal (SCCA) showed an equivalent response to treatment with cisplatin plus 5-fluorouracil (5-FU) versus carboplatin plus weekly paclitaxel. However, longer survival and less toxicity was observed in patients receiving carboplatin and paclitaxel, according to findings presented at the ESMO 2018 Congress in Munich, Germany.
Sheela Rao, Consultant Medical Oncologist Oncology at the Royal Marsden Hospital NHS Foundation Trust, London, United Kingdom reported results on behalf of an international team of investigators.
Although advanced SCCA is a rare disease, the incidence has risen by 2% per year over the past decade. Furthermore, medical need exists for this disease where, there currently is no consensus on management, leaving SCCA patients with poor overall survival (OS).
Prior to InterAACT, no randomised trial in SCCA had been completed. InterAACT was a randomised phase II, selection trial with pick the winner design that aimed to establish a standard of care by comparing combined fluoropyrimidine plus platinum agents, which was often considered standard first-line therapy with taxanes, which have shown activity in this patient population. Patients were enrolled from more than 50 centres located internationally.
From 2014 to 2017, the InterAACT investigators enroled 91 patients with inoperable locally recurrent or metastatic treatment-naïve, advanced SCCA.
The patients were equally randomised to receive cisplatin at 60 mg/m2 on day one of a 21 day cycle plus 5-FU at 1000 mg/m2 over 24 hours on day one 4 times during the cycle, or to be treated with carboplain at AUC 5 on day 1 every 28 days and paclitaxel at 80 mg/m2 on days 1, 8, 15 every 28 days. The patients were stratified according to performance status (PS), extent of disease, HIV status, and country. Overall, 67% of patients were women with a mean age of 61 years; 12% of patients had locally advanced, and 88% had metastatic disease.
The primary endpoint was response rate (RR), and secondary endpoints included progression-free survival (PFS), OS, toxicity, quality of life (QoL), and feasibility.
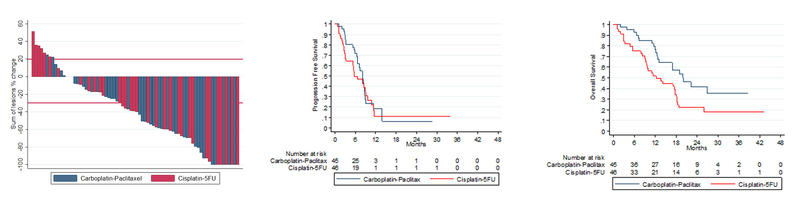
Fewer SAEs occurred with carboplatin plus paclitaxal
The RRs to treatment were 57.1% with cisplatin/5-FU compared to 59.0% with carboplatin/paclitaxel.
However, survival was prolonged with carboplatin/paclitaxel. Median PFS was 5.7 months for cisplatin/5-FU versus 8.1 months for carboplatin/paclitaxel (p = 0.375) and median OS with the respective treatments was 12.3 versus 20 months, hazard ratio [HR] 2.0 (p = 0.014).
Grade ≥3 toxicity was reported in 32 (76%) patients on cisplatin/5-FU and 30 (71%) patients on carboplatin/paclitaxel.
The incidence of serious adverse events (SAEs) was lower with carboplatin/paclitaxel; SAEs were reported in 62% of cisplatin/5-FU patients compared with 36% of patients receiving carboplatin/paclitaxel (p = 0.016).
Discussant points
Claus-Henning Köhne of the University Clinic Oncology and Haematology, North West German Cancer Center (NWTZ), who discussed the study findings, has started his talk by questioning the conclusions based on a small randomised phase II trial, powered for a “pick the winner” design for ORR. Prof. Köhne said that in 1986 a prospective randomised trial was demanded to establish surgery free treatment; however, this study has never been done and primary chemotherapy/radiotherapy is considered as a standard of care. He further discussed recent results with nivolumab for previously treated unresectable metastatic anal cancer. Prof. Köhne congratulated to all investigators for their important work in establishing carboplatin/paclitaxel in patients with inoperable, locally recurrent or metastatic treatment naive anal cancer and asked to continue with this multinational effort. He questioned whether the ‘New Kids on the Block’, such as immune checkpoint inhibitors, will add to this.
Conclusions
The authors pointed out that InterAACT is the first prospective randomised trial in patients with inoperable recurrent or metastatic SCCA.
They further note that, in this pick the winner designed trial, carboplatin/paclitaxel demonstrated a similar response rate as cisplatin/5-FU but less toxicity, and thus was declared the winner.
Importantly, this study has successfully demonstrated the feasibility of international collaboration in a rare cancer.
Moreover, these findings establish carboplatin/paclitaxel as a standard of care for first-line treatment of advanced SCCA, and suggest that carboplatin/paclitaxel may serve as a backbone for the addition of novel agents in future phase II/III trials.
Disclosure
This trial was sponsored by Cancer Research UK (CRUK), Biomedical Research Centre (BRC) Royal Marsden.
Reference
LBA21 – Rao S, Sclafani F, Eng C, et al. InterAACT: A multicentre open label randomised phase II advanced anal cancer trial of cisplatin (CDDP) plus 5-fluorouracil (5-FU) vs carboplatin (C) plus weekly paclitaxel (P) in patients (pts) with inoperable locally recurrent (ILR) or metastatic treatment naïve disease - An International Rare Cancers Initiative (IRCI) trial