Researchers reported data from the IMmotion151 (NCT02420821) phase III study at the ESMO 2018 Congress in Munich, Germany that confirmed previous findings of improved progression-free survival (PFS) with combined atezolizumab/bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (mRCC). The study also correlated molecular gene expression signatures with clinical outcomes, prognostic risk groups, and tumour histology.
Brian I. Rini of the Department of Solid Tumour Oncology, Taussig Cancer Institute Cleveland Clinic in Cleveland, United States of America and colleagues from other institutions conducted the IMmotion151 trial to confirm improved PFS with atezolizumab plus bevacizumab compared to sunitinib in patients whose tumours express PD-L1.
The investigators noted that biomarker analyses performed in a phase II study (IMmotion150) suggested that T effector (Teff) and interferon gamma, as well as Angiogenesis gene expression signatures (GEs) were associated with differential outcomes to atezolizumab plus bevacizumab and sunitinib.
They subsequently conducted prespecified genomic analyses to validate these GEs with clinical outcomes from 823 patients in IMmotion151 and also evaluated association of GEs with Memorial Sloan Kettering Cancer Centre (MSKCC) risk groups, and sarcomatoid histology.
IMmotion151 trial confirmed previous PFS results from phase II study
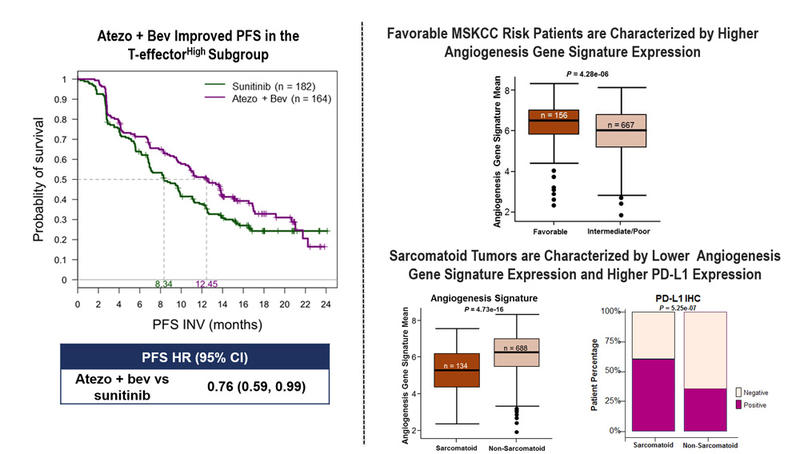
The IMmotion151 study met its co-primary endpoint of improved PFS with atezolizumab plus bevacizumab over sunitinib in PD-L1-positive patients across all MSKCC risk groups, hazard ratio (HR) 0.74; 95% confidence interval (CI), 0.57 - 0.96 (p = 0.02). Improved PFS was also observed in patients with sarcomatoid histology, HR 0.56; 95% CI, 0.38 - 0.83.
Molecular correlates differentiate clinical outcome observed with atezolizumab/bevacizumab versus sunitinib
Tumour molecular analyses showed that high Teff GE was associated with PD-L1 expression, as evaluatedby immunohistochemistry. High Teff GE also associated with longer PFS for atezolizumab plus bevacizumab compared to sunitinib, HR 0.76; 95% CI, 0.59 - 0.99.
In patients receiving sunitinib, high angiogenesis GE was associated with improved PFS, HR 0.59, 95% CI, 0.47 - 0.75; however high angiogenesis GE did not differentiate between atezolizumab plus bevacizumab versus sunitinib clinical activity, HR, 0.95; 95% CI, 0.75 - 1.19. Atezolizumab plus bevacizumab improved PFS versus sunitinib in the subset of patients with low angiogenesis GE, HR 0.68; 95% CI, 0.52 - 0.89.
Angiogenesis GE was found to be higher in favourable versus intermediate to poor MSKCC risk groups (p = 4.28e-06). PD-L1 prevalence was higher (63%) in sarcomatoid tumours, compared to 39% in non-sarcomatoid tumours. Angiogenesis GE was lower in sarcomatoid compared to non-sarcomatoid tumours (p = 4.73e-16)
Conclusions
Prospective evaluation of biomarkers in IMmotion 151 validated molecular signatures that could differentiate between clinical outcomes to the combination of VEGF inhibition provided by bevacizumab and PD-L1 inhibition provided by atezolizumab vs sunitinib in first-line treatment of patients with mRCC. These data also identified tumour genomic profiles that associated with prognostic risk groups and sarcomatoid histology.
The authors noted that biomarker findings from IMmotion151 advance our understanding of the biology of kidney cancer and may be used to inform future strategies enabling personalised therapy in patients with mRCC.
Disclosure
This trial was sponsored by F. Hoffmann-La Roche Ltd.
Reference
LBA 31 – Rini BI, Huseni M, Atkins MB, et al. Molecular correlates differentiate response to atezolizumab (atezo) + bevacizumab (bev) vs sunitinib (sun): results from a Phase III study (IMmotion151) in untreated metastatic renal cell carcinoma (mRCC).