Entrectinib is a CNS-active, potent inhibitor of all TRK proteins (TRKA/B/C) as well as ROS1 and ALK; NTRK gene fusions lead to the transcription of chimeric TRK proteins that have uncontrolled kinase function, which confers oncogenic signals across several tumour types.
Treatment with entrectinib induced responses that were durable in more than half of patients with solid tumours and neurotrophic tropomyosin receptor kinase (NTRK) rearrangements, according to findings reported at the ESMO 2018 Congress in Munich, Germany. Prof. George D. Demetri, director of the Centre For Sarcoma and Bone Oncology, Dana-Farber Cancer Institute in Boston, United States of America, presented an integrated efficacy and safety analysis from three phase I/II clinical trials of entrectinib: ALKA (EudraCT 2012-000148-88), STARTRK-1 (NCT02097810), and STARTRK-2 (NCT02568267).
The studies enrolled patients from more than 150 global sites in 15 countries with metastatic and/or locally advanced solid tumours that harbored NTRK-fusions confirmed by nucleic acid-based methods. Tumour assessment was done after 4 weeks of treatment and every 8 weeks thereafter. Scans were evaluated by blinded independent central review (BICR) using RECISTv1.1.
The primary endpoints of the trials were overall response rate (ORR) and duration of response (DoR) by BICR, and secondary endpoint included safety, progression-free survival (PFS), and overall survival (OS) in patients with and without baseline CNS disease.
This tumour agnostic efficacy analysis included 54 adult patients with a minimum of six months follow-up of advanced or metastatic NTRK-fusion positive solid tumours, comprising more than 19 histopathologies involving 10 general tumour types. Patients with baseline CNS metastasis were allowed to enrol.
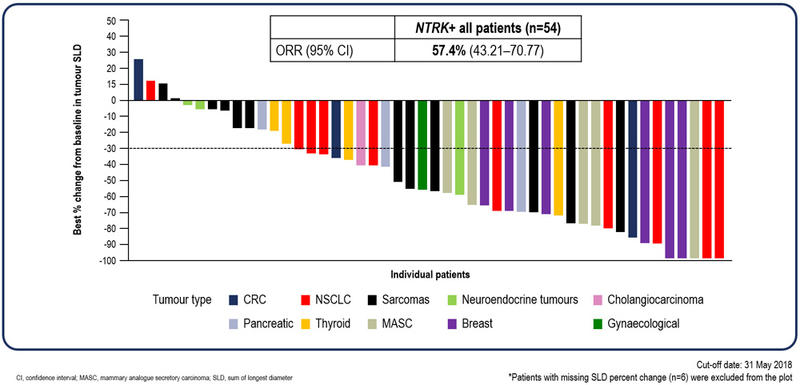
Complete responses were observed with entrectinib
According to BICR, the ORR was 57.4% (95% confidence interval [CI], 43.2 – 70.8%), which included 4 (7.4%) complete responses.
Responses were observed across all tumour types (see visual waterfall plot of data).
The median duration of response was 10.4 months (95% CI, 7.1 – not reached [NR]), median PFS was 11.2 months (95% CI, 8.0 – 14.9), all by BICR, and median OS was 20.9 months (95% CI, 14.9 – NR). The median follow-up for survival in these patients was 12.9 months.
Entrectinib has activity in patients with CNS metastasis
Data evaluation by investigator-assessed status of metastatic spread to the CNS at baseline revealed consistent responses both in patients without baseline CNS metastases (n=42; ORR 59.5%) and in patients with metastatic cancer to the CNS (n=12; ORR 50%). Additionally, intracranial response (IC-ORR 54.5%) in patients with CNS disease at baseline as assessed by BICR was observed to be similar to systemic response rates observed, including 3 intracranial complete responses.
The safety population included 355 patients treated with entrectinib across clinical trials. Overall, entrectinib was tolerable with a manageable safety profile. Most treatment-related adverse events (TRAEs) were grades 1–2 and were managed with dose reduction (27.3%) or dose interruption (25.4%); 3.9% of patients discontinued entrectinib due to TRAEs.
In 2017, the US Food and Drug Administration granted a breakthrough therapy designation to entrectinib for use as a treatment for adult and paediatric patients with NTRK-fusion positive, locally advanced or metastatic solid tumours who have either progressed following prior therapies or who are not eligible for standard therapies. The Priority Medicines (PRIME) designation from the European Medicines Agency has also been received in 2017, and Sakigake designation in Japan in March 2018.
Discussant Points
Dr Allan Jordan who discussed the study findings said that a new dataset focusses on the key TRK fusion population. He questioned if there is any contribution to efficacy from other fusions and if TRK is mutually exclusive with ALK and ROS. The PFS of around 11 months is encouraging. CNS activity is highly encouraging and offers significant potential clinical benefit.
Conclusions
According to the authors, this integrated analysis of global multicentre, clinical trials demonstrated that entrectinib was well tolerated overall and induced clinically meaningful, durable systemic responses in patients with NTRK-fusion positive solid tumours, across a variety of histologies, and in patients with and without CNS disease. This represents an advance in precision medicine, with entrectinib offering benefits for NTRK-fusion postive patients as a tumour agnostic targeted cancer therapy.
Disclosure
This trial was sponsored by Ignyta, Inc., a wholly owned subsidiary of F. Hoffmann-La Roche Ltd.
Reference
LBA17 – Demetri GD, Paz-Ares L, Farago AF, et al. Efficacy and Safety of Entrectinib in Patients with NTRK Fusion-Positive (NTRK-fp) Tumors: Pooled Analysis of STARTRK-2, STARTRK-1 and ALKA-372-001.