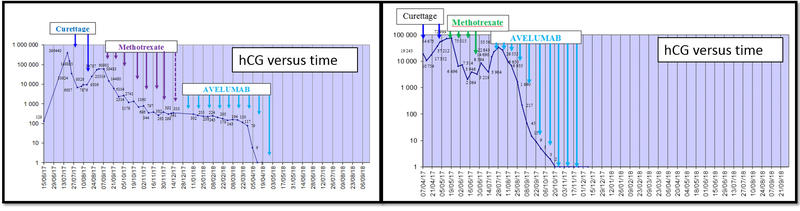
Treatment with the anti-PD-L1 monoclonal antibody, avelumab, provided normalisations of human chorionic gonadotrophin (hCG) in patients with chemotherapy-resistant gestational trophoblastic neoplasia (GTN) and was well-tolerated, according to preliminay results presented at the ESMO 2018 Congress in Munich, Germany.
Gestational trophoblastic diseases (GTD) represent a group of rare tumours that accounts for less than 1% of female reproductive system cancers. GTD involves abnormal growth of cells arising within the uterus following conception. GTD tumours grow in the cells that would normally develop into the placenta during pregnancy, rather than from cells of the uterus or cervix.
Benoit You, of the Hospices Civils de Lyon in Lyon, France and his colleagues from the French Gestational Trophoblastic Disease (GTD) Center led by Francois Golfier are conducting the academic phase II, multicentre TROPHIMMUN (NCT03135769 EudraCT Number: 2016-002322-37) trial to evaluate the efficacy of avelumab in patients with chemoresistant GTN. The rationale for this study is that PD-L1 is constitutively expressed in all GTN subtypes.1
By September 2018, cohort A of TROPHIMMUN enrolled 11 patients with GTN that had demonstrated resistance to monochemotherapy, and cohort B enrolled 4 patients with polychemotherapy resistant GTN. In cohort A, all patients had post-molar diagnosis of GTN based on hCG reincrease, 63% of patients had stage I, and 27% had stage III disease and 9% had stage IV; 45% of patients had FIGO score 1-4, and 55% had FIGO score 5-6 disease. All patients had shown prior resistances to methotrexate and 9% of patients had been resistant to actinomycine-D treatment. The median age of the patients was 33 (range 27 to 55) years.
All patients were treated with avelumab at 10 mg/kg every 2 weeks until normalisation of hCG levels was achieved, and for 3 additional cycles thereafter.
The primary endpoint of the ongoing 2 step Simon design study is the rate of patients with hCG normalisation. Achieving hCG normalisation is associated with a high chance of disease cure.
hCG normalisation was achieved by half of cohort A patients and decreases are being confirmed in the remaining patients
At ESMO 2018 Congress, Professor You presented the results of a pre-planned intermediate analysis of data from the first 6 patients treated with avelumumab in cohort A.
Among these patients, 3 patients attained normalised hCG and stopped treatment with no further indication of relapse within a 11.7 month follow-up. The other hCG declines observed in subsequently recruited patients remain to be confirmed.
The adverse events observed in all enrolled patients in cohort A has been in line with the favorable toxicity profile reported with this drug so far. Eight patients (73%) experienced 63 adverse events (AEs); 84 % grade 1 AE, 14.% grade 2 AE, including one case of hypothyroidism, and one grade 3, disease-related metrorragia.
Discussant Points
Mansoor Raza Mirza of the Nordic Society of Gynaecological Oncology and Rigshospitalet, Copenhagen University Hospital in Denmark who discussed the study findings said that it is about rare cancers in young patients with possible immune tolerance derived from pregnancy. In his talk, Dr Mirza pointed out to a lack of level 1 evidence in rare tumours and confounding factors for treatment-related outcomes. He congratulated for performing study in rare tumours and asked if the authors would consider to expand the study to a global level to achieve stronger evidence.
Conclusions
The investigators noted that TROPHIMMUN is the first clinical trial to report potential cures with a non-chemotherapy agent in patients with this rare cancer.
GTN patients that are resistant to chemotherapy generally receive standard treatment with historic single agent or polychemotherapy regimens, which are known to be effective in achieving 65% to 95% successful hCG normalisation. These regimens are also associated with high toxicity.
Since PD-L1 is expressed in GTN, there is a strong rational for investigating immunotherapies in GTN patients. The on-going translational research projects led by Pierre-Adrien Bolze, from the French GTD center, should help better select the patients who will benefit the most from avelumab.
The preliminary results from cohort A of the TROPHIMMUN trial suggest that avelumab might be effective and better tolerated than standard chemotherapy in patients with resistance to single chemotherapy.
Citation
1. Bolze PA, Patrier S, Massardier J et al. PD-L1 Expression in Premalignant and Malignant Trophoblasts From Gestational Trophoblastic Diseases Is Ubiquitous and Independent of Clinical Outcomes. Int J Gynecol Cancer 2017; 27(3): 554-561.
Disclosure
This investigator-initiated trial is sponsored by Hospices Civils de Lyon, with the support of Merck-Serono.
Reference
LBA35 – You BM, Bolze PA, Lotz J-P, et al. TROPHIMMUN, a 2 cohort phase II trial of the anti-PD-L1 monoclonal antibody avelumab in chemo-resistant gestational trophoblastic neoplasia (GTN) patients: preliminary outcomes in cohort A.