Survival with first-line pembrolizumab in patients with PD-L1 combined positive score ≥1 (CPS ≥1) advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma was non-inferior to chemotherapy, whereas patients with higher levels of PD-L1 and patients with microsatellite instability–high (MSI-H) tumours showed encouraging benefit with pembrolizumab, according to findings from the phase III KEYNOTE-062 study presented at the ESMO Congress 2019 in Barcelona, Spain.
Kohei Shitara, Gastrointestinal Oncology, National Cancer Centre Hospital in Kashiwa, Japan presented results on behalf of a team of investigators from the phase III KEYNOTE-062 (NCT02494583) study of pembrolizumab or pembrolizumab plus chemotherapy compared to standard chemotherapy in the first-line setting for patients with HER2-negative, advanced G/GEJ cancer and a PD-L1 CPS ≥1 and CPS ≥10.
Following 1:1:1 randomisation 256 patients received pembrolizumab at 200 mg every 3 weeks for up to 2 years, 257 patients received pembrolizumab at the same dose plus chemotherapy comprising cisplatin at 80 mg/m2 plus 5-FU 800 mg/m2 per day on days 1 to 5 every 3 weeks or capecitabine at 1000 mg/m2 twice daily on days 1 to 14 every 3 weeks per local guidelines, and 250 patients received placebo plus chemotherapy every 3 weeks. Of the 763 patients randomised, 281 had PD-L1 CPS ≥10.
The primary endpoints were overall survival (OS) in the CPS ≥1 and CPS ≥10 for pembrolizumab plus chemotherapy compared to chemotherapy and pembrolizumab versus chemotherapy, as well as progression-free survival (PFS) per RECIST v1.1 by central review in the CPS ≥1 for pembrolizumab plus chemotherapy compared to chemotherapy. The secondary endpoint was objective response rate (ORR) per RECIST v1.1 by central review in the CPS ≥1 for pembrolizumab plus chemotherapy compared to chemotherapy.
As of the cut-off date of 26 March 2019 the median follow-up was 11.3 months.
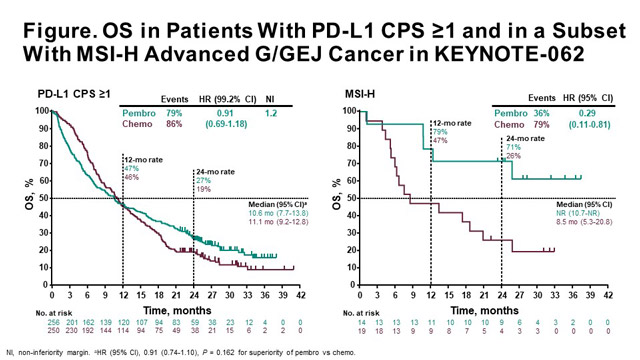
OS with pembrolizumab was non-inferior to chemotherapy per prespecified margins
Regarding the primary endpoints, in the CPS ≥1, median OS with pembrolizumab was 10.6 months (95% confidence interval [CI], 7.7-13.8) compared to 11.1 months (95% CI, 9.2-12.8) with chemotherapy (hazard ratio [HR] 0.91; 99.2% CI, 0.69-1.18; non-inferiority margin = 1.2). Therefore, this study met the primary endpoint to show non-inferiority. In the CPS ≥10, pembrolizumab prolonged OS, but wasn’t tested per analysis plan; median OS was 17.4 months with pembrolizumab compared to 10.8 months with chemotherapy (HR 0.69; 95% CI, 0.49-0.97). Median OS with pembrolizumab plus chemotherapy was 12.5 months (95% CI, 10.8-13.9) versus 11.1 months (95% CI, 9.2-12.8) with chemotherapy (HR 0.85; 95% CI, 0.70-1.03; p = 0.046).
For the pembrolizumab monotherapy versus chemotherapy comparison, median PFS was 2.0 months (95% CI, 1.5-2.8) versus 6.4 months (95% CI, 5.7-7.0), respectively (HR 1.66; 95% CI, 1.37-2.01). Median PFS was 6.9 months (95% CI, 5.7-7.3) with pembrolizumab plus chemotherapy versus 6.4 months (95% CI, 5.7-7.0) with chemotherapy (HR 0.84; 95% CI, 0.70-1.02; p = 0.039).
Exploratory analysis shows substantial benefit with pembrolizumab
In an exploratory analysis of 50 patients with MSI-H tumours, median OS was not reached (NR) in both pembrolizumab arms; for the comparison of pembrolizumab versus chemotherapy, median OS was NR (95% CI, 10.7-NR) versus 8.5 months (95% CI, 5.3-20.8), respectively (HR 0.29; 95% CI, 0.11-0.81). Median OS was NR (95% CI, 3.6-NR) with pembrolizumab plus chemotherapy compared to 8.5 months (95% CI, 5.3-20.8) with chemotherapy (HR 0.37; 95% CI 0.14-0.97).
PFS was also prolonged in both pembrolizumab arms in patients with MSI-H tumours; for pembrolizumab versus chemotherapy, median PFS was 11.2 months (95% CI, 1.5-NR) versus 6.6 months (95% CI, 4.4-8.3), respectively (HR 0.72; 95% CI, 0.31-1.68). Median PFS was NR (95% CI, 3.6-NR) with pembrolizumab plus chemotherapy versus 6.6 months (95% CI, 4.4-8.3) with chemotherapy (HR 0.45; 95% CI, 0.18-1.11).
Pembrolizumab provided a greater response in this group; the ORR with pembrolizumab versus chemotherapy was 57.1% versus 36.8%, and ORR with pembrolizumab plus chemotherapy versus chemotherapy was 64.7% versus 36.8%, respectively. The median durations of response were 21.2 months with pembrolizumab and NR with pembrolizumab plus chemotherapy versus 7.0 months with chemotherapy.
Grade 3-5 drug-related adverse events (AEs) occurred in 17% of patients in the pembrolizumab arm and 73% patients receiving pembrolizumab plus chemotherapy versus 69% of patients on chemotherapy.
High compliance rates observed for both QoL scales with pembrolizumab and chemotherapy
Eric Van Cutsem, Digestive Oncology, University Hospitals and University of Leuven (KUL), Belgium presented the health-related quality of life (HRQoL) analysis of the KEYNOTE-062 study.
Professor Van Cutsem and colleagues evaluated HRQoL using the EORTC QLQ-C30 and EORTC QLQ-STO22 scales, which were administered at baseline, weeks 3, 6, 9, and 12, and every 6 weeks thereafter up to one year or end of treatment, as well as at the 30-day post-treatment discontinuation follow-up visit. The analysis included patients receiving one or more doses of study treatment and completing ≥1 HRQoL assessment. QoL data were described as the least square mean (LSM) score change from baseline to prespecified week 18. Time to deterioration (TTD) was defined as ≥10-point decline from baseline and was assessed by Kaplan-Meier method and Cox regression model.
The HRQoL analysis included 495 patients with PD-L1 CPS ≥1; of these 252 received pembrolizumab and 243 received chemotherapy. Compliance at week 18 was similar in both treatment arms for both scales; 87.9% of patients on pembrolizumab and 81.9% of patients completed the QLQ-C30, whereas 87.9% versus 81.3% completed the QLC-STO22, respectively.
No significant difference in LSM score change from baseline to week 18 between arms was observed in global health status (GSH) QoL; the LSM score change from baseline was –0.16 (95% CI, –5.01 to 4.69; p = 0.948).
Significantly longer TTD was observed for the nausea/vomiting subscale in QLQ-C30 with pembrolizumab compared to chemotherapy (HR 0.61; 95% CI, 0.44-0.85; p = 0.003).
In other measurements, most of the items of the QLQ-C30 and QLQ-STO22 subscales showed comparable but not statistically significant worsening in both treatment arms. The TTD comparisons for GSH QoL between pembrolizumab and chemotherapy were similar (HR 0.96; 95% CI, 0.67-1.38; p = 0.826), as were the appetite loss subscale in QLQ-C30 (HR 0.83; 95% CI, 0.58-1.20; p = 0.314), and pain subscale in QLQ-STO22 (HR 1.22; 95% CI, 0.78-1.91; p = 0.381) in the pembrolizumab and chemotherapy arms, respectively.
Conclusions
The authors concluded that OS for first-line pembrolizumab was non-inferior to chemotherapy in patients with advanced gastric cancer, and clinically meaningful improved OS was observed with pembrolizumab in patients with CPS ≥10.
Pembrolizumab plus chemotherapy did not demonstrate superior OS or PFS in patients with CPS ≥1 and OS in patients with CPS ≥10. Clinical benefit was substantially enhanced in a small subset of patients with MSI-H tumours.
The safety profile was more favourable for pembrolizumab compared to both arms containing chemotherapy. In the KEYNOTE-062 study, HRQoL was similar between pembrolizumab and chemotherapy in the first-line treatment for patients with advanced G/GEJ adenocarcinoma and CPS ≥1.
The authors acknowledge that some content from the abstract LBA44 has already been presented at ASCO 2019 Annual Meeting. However, data on OS in patients with MSI-H tumours and on QoL are first time presented at ESMO 2019 Congress.
Disclosure
The KEYNOTE-062 study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA.
References
LBA44 – Shitara K, Van Cutsem E, Bang Y-J, et al. Pembrolizumab With or Without Chemotherapy vs Chemotherapy in Patients With Advanced G/GEJ Cancer (GC) Including Outcomes According to Microsatellite Instability-High (MSI-H) Status in KEYNOTE-062.
LBA45 – Van Cutsem E, Valderrama A, Bang Y-J, et al. Health-Related Quality of Life (HRQoL) Impact of Pembrolizumab (P) Versus Chemotherapy (C) as First-Line (1L) Treatment in PD-L1–Positive Advanced Gastric or Gastroesophageal Junction (G/GEJ) Adenocarcinoma.