Patients with newly diagnosed ovarian cancer demonstrated significantly improved progression-free survival (PFS) with addition of olaparib to bevacizumab maintenance treatment, as compared to standard of care bevacizumab, following platinum-based first-line chemotherapy according to phase III study findings presented at the ESMO Congress 2019 in Barcelona, Spain.
Isabelle L. Ray-Coquard of the Medical Oncology, Centre Léon Bérard in Lyon, GINECO group France presented findings from the PAOLA-1/ENGOT-ov25 trial, which evaluated the efficacy and safety of olaparib in tablet formulation in patients with newly diagnosed advanced ovarian cancer.
PAOLA-1/ENGOT-ov25 was a randomised, double-blind, international phase III trial (NCT02477644) that enrolled patients with newly diagnosed, FIGO stage III–IV, high-grade serous or endometrioid ovarian cancer, fallopian tube or primary peritoneal cancer. This Phase III study was sponsored by the Association de Recherche sur les CAncers dont GYnécologiques (ARCAGY)-Research on behalf of GINECO, lead group for the PAOLA-1 trial of the European Network for Gynecological Oncological Trial Groups (ENGOT).
Patients in clinical complete or partial response following platinum-based chemotherapy plus bevacizumab were randomised 2:1 to receive oral olaparib at 300 mg twice daily for up to 24 months or placebo plus bevacizumab at 15 mg/kg on day one every 3 weeks for 15 months, which included doses received during chemotherapy. The patients were stratified by first-line treatment outcome and tumour BRCAmutation.
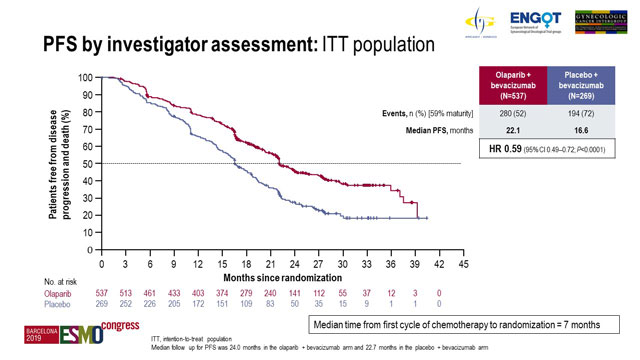
The primary endpoint was investigator-assessed PFS according to RECIST v1.1 in the intent-to-treat (ITT) population.
The median follow-up was 24.0 months for 537 patients in the olaparib arm and 22.7 months for 269 patients in the placebo arm.
Analysis of the data at 59% maturity demonstrated median PFS of 22.1 months with olaparib compared to 16.6 months with placebo (hazard ratio [HR] 0.59; 95% confidence interval [CI], 0.49-0.72; p < 0.0001).
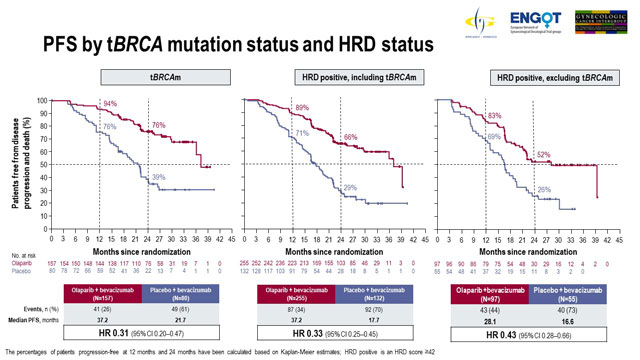
Patients with tumour BRCA mutation and HRD-positive patients derived superior PFS benefit with olaparib
The results of prespecified subgroup analyses were also presented. Regarding patients stratified by BRCA mutation status, 237 patients had tumour BRCA mutation and 569 patients had non-tumour BRCA mutation.
Among patients with tumour BRCA mutation, median PFS with olaparib treatment was 37.2 months compared to 21.7 months with placebo (HR 0.31; 95% CI 0.20-0.47). Patients with non-tumour BRCA mutation demonstrated median PFS of 18.9 months with olaparib versus 16.0 months with placebo (HR 0.71; 95% CI, 0.58-0.88).
Analysis according to HRD status showed that 387 patients were HRD-positive; of these 152 were non-tumour BRCA. HRD status was negative or unknown for 419 patients.
In HRD-positive patients, median PFS with olaparib was 37.2 months compared to 17.7 months with placebo (HR 0.33; 95% CI, 0.25-0.45). Median PFS in HRD-positive non-tumour BRCA patients treated with olaparib was 28.1 months versus 16.6 months with placebo (HR 0.43; 95% CI, 0.28-0.66). Olaparib had little effect in patients who were not HRD-positive; in patients having negative or unknown HRD status, median PFS was 16.9 months versus 16.0 months with olaparib versus placebo (HR 0.92; 95% CI, 0.72-1.17).
Data regarding PFS2 were not mature at the time of this analysis.
Grade ≥3 adverse events (AEs) occurred in 57% versus 51% of patients receiving olaparib and placebo. The most commonly reported AEs were hypertension (19% versus 30%) and anaemia (17% versus <1%).
There were five fatal treatment emergent AEs, one with olaparib and 4 in the placebo arm. Dose interruptions occurred in 54% versus 24%, dose reductions occurred in 41% versus 7%, and treatment discontinuation occurred in 20% versus 6% of patients receiving olaparib and placebo, respectively.
Although treatment interruptions, reductions, discontinuations occurred more often with olaparib no clinically meaningful difference in health-related quality of life was observed between treatment arms.
Discussant points
Ana Oaknin of the Vall d´Hebron Institute of Oncology, Barcelona, Spain who discussed the study findings divided her talk in order to cover next important questions: is the benefit of adding a PARPi as maintenance therapy to first-line treatment clinically meaningful enough to justify its use as a new standard of care, can biomarker status guide in the selection of patients who benefit most, could the different control arms in the trials have modified the outcomes, can we hypothesize which sequence of therapies is the best for our patients, and are there any toxicity concerns about the use in first-line therapy. In PAOLA-1, the rate of AEs leading to treatment discontinuation was 20%: this is the highest figure reported across PARPi trials. However, there was no impact in quality of life. Dr Oaknin concluded that we are witnessing a paradigm shift in the first-line treatment for advanced ovarian cancer patients.
Conclusions
PAOLA-1/ENGOT-ov25 met its primary objective, demonstrating a statistically significant and clinically meaningful improvement in PFS in the ITT population when olaparib compared with placebo was added to first-line standard of care bevacizumab maintenance treatment. The PFS benefit in subgroups of patients with tumour BRCA mutation and in patients that were HRD-positive was substantial.
According to the authors, this is the first phase III trial to evaluate efficacy and safety of a PARP inhibitor plus bevacizumab as first-line maintenance therapy in advanced ovarian cancer not restricted by surgical outcome or BRCA mutation status.
Disclosure
This study was funded by ARCAGY Research, AstraZeneca, Merck & Co., Inc. and Hoffmann-La Roche.
Reference
LBA2_PR - Ray-Coquard IL, Pautier P, Pignata S, et al. Phase III PAOLA-1/ENGOT-ov25 trial: Olaparib plus bevacizumab (bev) as maintenance therapy in patients (pts) with newly diagnosed, advanced ovarian cancer (OC) treated with platinum-based chemotherapy (PCh) plus bev.