Newly presented data from the phase III FLAURA (NCT02296125) study builds on earlier reported findings of significantly improved progression-free survival (PFS) with first-line osimertinib over current standard-of-care (SoC) therapies in patients with advanced non-small cell lung cancer (NSCLC) harbouring epidermal growth factor receptor (EGFR) mutations. New data supporting the use of osimertinib in the first-line setting in patients with NSCLC and EGFR mutations were presented at the European Lung Cancer Congress (ELCC) 2018, held 11 to 14 April in Geneva, Switzerland.
Osimertinib is currently approved in Europe for the treatment of patients with advanced NSCLC with the EGFR T790M mutation.
Professor David Planchard, Department of Medical Oncology, Institut Gustave Roussy in Villejuif, France reported exploratory post-progression outcomes on behalf of the FLAURA investigators. This study compared osimertinib with SoC EGFR tyrosine kinase inhibitor (TKI) treatment consisting of gefitinib or erlotinib in the first-line setting in patients with EGFR mutation-positive (exon 19 deletion or L858R) locally advanced or metastatic NSCLC.
In the FLAURA trial, 556 patients were randomised equally to oral osimertinib at 80 mg once daily (n = 279) or to SoC EGFR-TKI (n = 277), consisting of either oral gefitinib at 250 mg once daily or oral erlotinib at 150 mg once daily. Patients continued to receive treatment until disease progression, the development of unacceptable side effects, or withdrawal of consent. Treatment beyond disease progression with subsequent therapy was per investigator discretion and patients on SoC EGFR-TKI were allowed to cross-over to osimertinib following documentation of T790M-positive mutation status post progression.
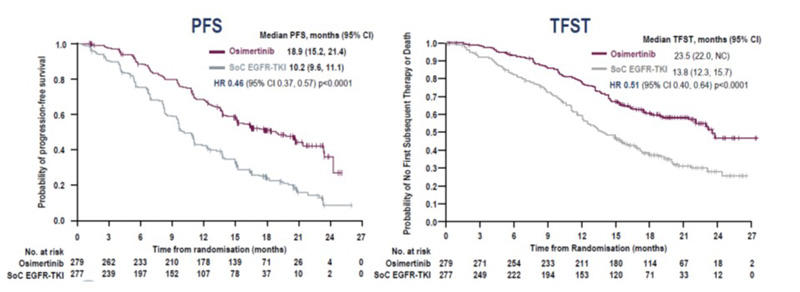
Treatment with osimertinib in the FLAURA trial showed significantly improved PFS compared to patients on SoC EGFR-TKI, hazard ratio [HR] 0.46, 95% confidence interval [CI] 0.37, 0.57 (p < 0.001);1 interim overall survival (OS) data were encouraging but not formally statistically significant, HR 0.63; 95% CI 0.45, 0.88 (p = 0.007).
Analysis of post-progression data showed fewer osimertinib-treated patients discontinued treatment, experienced progression or died
As of the data cut-off on 12 June 2017, 138 (49%) patients in the osimertinib treatment arm and 213 (77%) patients in the SoC EGFR-TKI arm had discontinued study treatment. First subsequent therapy (FST) was received by 82 (29%) patients in the osimertinib arm versus 129 (47%) in the SoC EGFR-TKI arm. In the respective arms, 29 (10%) versus 97 (35%) patients received EGFR-TKI-containing therapy (55 [20%] were treated with subsequent osimertinib in the SoC EGFR-TKI) and 46 (16%) versus 27 (10%) patients received platinum chemotherapy-containing treatment.
With osimertinib the median time to discontinuation of study treatment or death was 20.8 months (95% CI 17.2, 24.1) compared to 11.5 months (95% CI 10.3, 12.8) with SoC EGFR-TKI. The median time to discontinuation of any EGFR-TKI (study treatment and subsequent EGFR-TKI, not interrupted by non-EGFR-TKI therapy) or death was 23.0 months (95% CI 19.5, not calculable [NC]) with osimertinib versus 16.0 months (95% CI 14.8, 18.6) with SoC EGFR-TKI.
In the osimertinib and SoC EGFR-TKI arms, 91 (67%) and 206 (74%) patients, respectively, remained on study treatment for at least 7 days following investigator-assessed progression. Median duration on study treatment post-progression in the respective arms was 8.1 weeks (95% CI 6.3, 12.3) versus 7.0 weeks (95% CI 5.9, 8.1).
Time-to-event post-progression endpoints all favoured osimertinib
The median time to first subsequent therapy (TFST) or death was 23.5 (95% CI 22.0, NC) versus 13.8 months (95% CI 12.3, 15.7) in the osimertinib and SoC EGFR-TKI arms respectively, HR 0.51 (95% CI 0.40, 0.64, p < 0.0001).
In the osimertinib versus SoC EGFR-TKI arms, 73 (26%) versus 106 (38%) patients experienced a second progression or died. Median time from randomisation to second progression on subsequent treatment (PFS2) was NC (95% CI 23.7, NC) versus 20.0 months (95% CI 18.2, NC), respectively, HR 0.58 (95% CI 0.44, 0.78, p = 0.0004).
Seventy-four (27%) osimertinib versus 110 (40%) SoC EGFR-TKI patients started second subsequent therapy (SST) or died; 24 (9%) versus 39 (14%) started SST, and 50 (18%) versus 71 (26%) patients died, respectively.
The median time to SST (TSST) or death was NC for the osimertinib arm versus 25.9 months (95% CI 20.0, NC) or the SoC EGFR-TKI arm, HR 0.60 (95% CI 0.45, 0.80, p = 0.0005).
Dr. Tetsuya Mitsudomi, who discussed the abstract data, said that as a study endpoint the OS is golden standard, unambiguous, independent of bias-prone variables. However, it is impractical because of the length, cost, and the size of clinical trials. In term of capturing the impact of subsequent therapies, it is both beneficial and detrimental, but it does not take into account the contribution of subsequent therapies by treatment arms. The PFS is easier to measure but depends on bias-prone variables such as frequency of assessment or definition of progression. Furthermore PFS benefit is often not translated into OS benefit. PFS2 is recommended for use when OS cannot be measured for clinical and financial reasons to assess clinical benefit of agents that do not induce responses, effect of maintenance therapy, impact of crossover on OS assessment, and whether the experimental therapy positively or negatively affects efficacy in the subsequent therapy (spill-over effect).
In FLAURA, post-progression outcome endpoints (TFST, PFS2, TSST) are more likely to be associated with OS. All post-progression outcome endpoints were prolonged with osimertinib vs SoC EGFR-TKI, providing greater confidence in the interim OS data. In addition, time to discontinuation of TKI was longer in the osimertinib arm, even with reasonable crossover from the SoC to osimertinib. These data further support the first-line use of osimertinib for EGFR-mutated NSCLC patients as (one of) the best treatment(s).
Similar quality of life data from FLAURA observed with osimertinib and SoC
During ELCC 2018, Natasha Leighl, Medical Oncology, Princess Margaret Hospital, Toronto, Canada presented compelling quality of life (QoL) data showing that the improvement in QoL with osimertinib was equivalent to that provided by the SoC. Patients in FLAURA reported similar improvements in cough, dyspnoea, chest pain, appetite loss, and fatigue.
More than 60% of patients in both treatment cohorts completed QoL questionnaires at all time points, including the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire Core 30 items (EORTC-QLQ-C30) at baseline and every 6 weeks thereafter, and the Lung Cancer 13 items (QLQ-LC13) at baseline, then weekly for 6 weeks followed by every 3 weeks. Scoring ranges from 0 to 100 with higher scores representing greater symptom burden; a difference ≥10-points was considered clinically relevant. The pre-specified key symptoms included cough, dyspnoea, chest pain, fatigue and appetite loss.
Clinically relevant improvements in cough were reported by both treatment arms
The mean baseline scores were equivalent in the osimertinib and SoC arms for the key symptoms: cough (32.8 versus 33.5), dyspnoea (22.5 versus 25.0), chest pain (19.5 versus 20.8), fatigue (32.2 versus 35.8) and appetite loss (22.7 versus 25.6).
Key symptoms improved in both treatment arms from baseline until randomised treatment discontinuation; however, this was clinically relevant only for cough in the osimertinib arm (-10.14). Change in cough for SoC was -8.18. Changes from baseline in other key symptoms with osimertinib versus SoC, respectively, included chest pain (-6.8 versus -3.9), appetite loss (-5.8 versus -4.4), fatigue (-3.3 versus -3.3), and dyspnoea (-3.2 versus -1.2). With the exception of chest pain (p = 0.021), there were no significant differences between the osimertinib and SoC arms.
Improved QLQ-C30 functional and global health/quality of life scores were also reported that showed no clinically relevant differences between cohorts.
Median time from randomisation to the first recorded clinically relevant deterioration of key lung cancer symptoms was similar between the two treatment arms.
Dr. Simon Ekman, who discussed the poster data, said that the compliance was above 70% at most of the time points in both treatment arms; at baseline for QLQ-LC13, 90.8% in osimertinib arm and 92.0% in SoC arm and for QLQ-C30, 95.2% in osimertinib arm and 94.1% in SoC arm. Key patient-reported symptoms have been improved in both treatment arms from baseline until randomised treatment discontinuation; however, it was clinically relevant only for cough in osimertinib arm. He pointed out that the degree of change perceived to be clinically significant could well differ from population to population and from patient to patient. Minor benefits for osimertinib were observed in term of chest pain, emotional, cognitive and social functioning. Time to deterioration of key symptoms was similar between treatment arms.
Dr. Ekman underlined that it is very important to study PROs, especially for TKIs as longer treatment periods and daily dosing potentially burdening the patients with drug-related adverse events. More research is needed on patients’ experiences captured with standardised measures, integration and report alongside more traditional trial outcome. In practice regular assessment of PROs would permit more timely, supportive interventions to reduce symptoms and side effects. It is important to encourage reporting PROs alongside PFS and other more traditional outcomes, not published in a separate paper.
Conclusions: The FLAURA authors concluded that the previously reported PFS benefit with osimertinib versus SoC was maintained throughout all time-to-event post-progression endpoints: PFS HR 0.46, TFST HR 0.51, PFS2 HR 0.58, and TSST 0.60. This step-wise increase of the statistically significant hazard ratios HRs provides confidence in the interim OS data.
Dr. Leighl noted that the key patient-reported symptoms improved in both treatment arms from baseline until treatment discontinuation with the improvement in cough in the osimertinib arm being clinically relevant.
Disclosure: Funding from Astra Zeneca for the FLAURA trial was disclosed.
Citation
1Soria JC et al. N Engl J Med doi: 10.1056/NEJMoa1713137.
Reference
128O – Planchard D, Boyer M, Lee J-S, et al. Osimertinib vs standard of care (SoC) EGFR-TKI as first-line therapy in patients (pts) with untreated EGFRm advanced NSCLC: FLAURA post-progression outcomes.
139PD - Leighl N, Karaseva N, Nakagawa K, et al. Patient-reported outcomes from FLAURA: Osimertinib versus standard of care (SoC) epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) in patients with EGFR-mutated advanced non-small cell lung cancer (NSCLC).