LUGANO-COPENHAGEN – Ipilimumab as adjuvant therapy significantly improves overall survival in patients with high risk stage III melanoma, according to the EORTC 18071 phase III trial results presented for the first time today at the ESMO 2016 Congress in Copenhagen.
“Ipilimumab is an immune checkpoint inhibitor that blocks cytotoxic T-lymphocyte antigen-4 (CTLA-4),” said lead author Professor Alexander Eggermont, director general, Institut Gustave Roussy, Villejuif, France. “It was approved in 2011 for first-line treatment of advanced melanoma in the US and Europe. The next question was its utility in the adjuvant setting.”
The EORTC 18071 phase III trial evaluated ipilimumab as adjuvant therapy for patients with high risk stage III melanoma. During 2008 to 2011, 951 patients were randomised to ipilimumab or placebo. Interferon was not used as the comparator because in Europe it is not routinely used nor accepted as a standard of care.
As reported in 2015, the study met its primary endpoint after a median follow up of 2.3 years, with ipilimumab significantly improving recurrence-free survival. The drug was subsequently approved by the US Food and Drug Administration as adjuvant therapy for stage III melanoma.
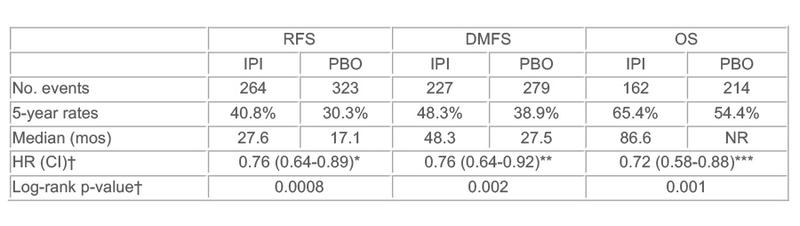
Now, at 5.3 years median follow up, the impact on overall survival is reported and represents a 28% reduction of the relative risk of death (hazard ratio 0.72, p=0.001). There was consistency across all endpoints with hazard ratios of 0.76 for recurrence-free survival and distant metastases-free survival (p<0.001 and p=0.002). In absolute terms, the overall survival rate at five years was 11% higher in the ipilimumab arm (65%) than in the placebo arm (54%).
Ipilimumab is known to create immune-related adverse events. At 5.3 years, there were no additional toxicities or deaths since the initial report at 2.3 years. The most important grade 3-4 adverse events were gastrointestinal (16%), hepatic (11%) and endocrine (8%). These were managed by established algorithms and usually resolved within 4-8 weeks. Endocrine adverse events took much longer to resolve or required permanent hormonal replacement therapies.
Eggermont said: “Ipilimumab adjuvant therapy brings a significant improvement of overall survival and has a favourable risk-benefit ratio. It clearly represents a serious option for patients with stage III melanoma.”
Commenting on the results, Dr Olivier Michielin, head of Personalised Analytical Oncology, CHUV, Lausanne, Switzerland, said: “This was the first attempt to use checkpoint blockade in the adjuvant setting of melanoma. The effect was a 28% reduction in the risk of death, which is statistically and clinically significant, and an 11% absolute gain in overall survival at five years.”
“This was also an important scientific discovery,” added Michielin. “Ipilimumab works by stimulating the immune system against tumour antigens. In the adjuvant setting there is microscopic residual disease and, until now, it was not clear if there was a sufficient amount of antigens to trigger a response.”
He continued: “The risks and benefits of this option should now be discussed with our patients. The toxicity is not negligible and patients need to be aware of the adverse event profile. The 10 mg/kg regimen used in the trial is associated with potentially severe toxicities and should be reserved for experienced centres.”
Michielin concluded: “This trial represents an important milestone in the treatment of melanoma. These results open the door for other studies based on checkpoint blockade to try and improve cure rates in the adjuvant setting of melanoma as well as other disease types. We are currently waiting for the results of several trials including EORTC 1325 which is investigating pembrolizumab, a PD-1 checkpoint blocking antibody, compared to placebo in the adjuvant setting.”
-END-
Notes to Editors
References
Abstract LBA2_PR – ‘Ipilimumab (IPI) vs placebo (PBO) after complete resection of stage III melanoma: final overall survival results the EORTC 18071 randomized, double-blind, phase 3 trial’ will be presented by Professor Alexander Eggermont during Presidential Symposium 1 on Saturday 8 October, 16:30 to 18:00 hours (CEST).
Eggermont AM, Chiarion-Sileni V, Grob JJ, Dummer R, Wolchok JD, Schmidt H, Hamid O, Robert C, Ascierto PA, Richards JM, Lebbé C, Ferraresi V, Smylie M, Weber JS, Maio M, Konto C, Hoos A, de Pril V, Gurunath RK, de Schaetzen G, Suciu S, Testori A. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16(5):522–530. doi: 10.1016/S1470-2045(15)70122-1.
Disclaimer
This press release contains information provided by the authors of the highlighted abstracts and reflects the content of those abstracts. It does not necessarily reflect the views or opinions of ESMO and ESMO cannot be held responsible for the accuracy of the data. Commentators quoted in the press release are required to comply with the ESMO Declaration of Interests policy and the ESMO Code of Conduct.
About the European Society for Medical Oncology
ESMO is the leading professional organisation for medical oncology. Comprising more than 13,000 oncology professionals from over 130 countries, we are the society of reference for oncology education and information. We are committed to supporting our members to develop and advance in a fast-evolving professional environment.
Founded in 1975, ESMO has European roots and a global reach: we welcome oncology professionals from around the world. We are a home for all oncology stakeholders, connecting professionals with diverse expertise and experience. Our educational and information resources support an integrated, multi-professional approach to cancer treatment. We seek to erase boundaries in cancer care as we pursue our mission across oncology, worldwide.
Abstract for LBA2_PR
Ipilimumab (IPI) vs placebo (PBO) after complete resection of stage III melanoma: final overall survival results from the EORTC 18071 randomized, double-blind, phase 3 trial
A.M.M. Eggermont1, V. Chiarion-Sileni2, J-J. Grob3, R. Dummer4, J.D. Wolchok5, H. Schmidt6, O. Hamid7, C. Robert8, P.A. Ascierto9, J.M. Richards10, C. Lebbé11, V. Ferraresi12, M. Smylie13, J.S. Weber14, C. Taitt15, V. de Pril16, G. de Schaetzen17, S. Suciu17, A. Testori18
1Oncological Surgery, Gustave Roussy Cancer Campus Grand Paris, Villejuif, France, 2Department of Dermatology, IOV-IRCCS, Melanoma Oncology Unit, Padua, Italy, 3Department of Dermatology & Skin Cancer, Aix-Marseille University, Hôpital de La Timone APHM, Marseille, France, 4Department of Dermatology, University of Zürich Hospital, Zurich, Switzerland, 5Melanoma and Immunotherapeutics Service, Memorial Sloan-Kettering Cancer Center, New York, NY, USA, 6Department of Dermatology, Aarhus University Hospital, Aarhus, Denmark, 7Clinical Research, The Angeles Clinic and Research Institute, Los Angeles, CA, USA, 8Dermatology Unit, Gustave Roussy Cancer Campus Grand Paris, Villejuif, France, 9Melanoma Cancer Immunotherapy and Innovative Therapy Unit, Istituto Nazionale Tumori Fondazione "G. Pascale", Naples, Italy, 10Division of Hematology/Oncology, Oncology Specialists S.C., Park Ridge, IL, USA, 11Department of Dermatology, APHP, Dermatology and CIC departments Hôpital Saint Louis, University Paris 7 INSERM U976, Paris, France, 12Department of Medical Oncology, Istituti Fisioterapici Ospitalieri, Rome, Italy, 13Department of Oncology, Cross Cancer Institute, Edmonton, AB, Canada, 14Department of Medicine, H Lee Moffitt Cancer Center (currently at Perlmutter Cancer Center at NYU-Langone Medical Center), Tampa, FL, USA, 15Worldwide Medical, Bristol-Myers Squibb, Princeton, NJ, USA, 16Worldwide Medical, Bristol-Myers Squibb, Braine-l’Alleud, Belgium, 17Melanoma and Children Leukemia Groups, EORTC Headquarters, Brussels, Belgium, 18Divisione Melanoma e Sarcomi Muscolo-Cutanei, European Institute of Oncology, Milan, Italy
Background: IPI has been an approved treatment for stage III melanoma in the US since 2015, based on a significant improvement in recurrence-free survival (RFS; hazard ratio [HR] 0.75; P=0.0013) (Eggermont et al, Lancet Oncol, 2015). Here, we report the impact of IPI on overall survival (OS) and distant metastasis-free survival (DMFS).
Methods: In this trial, eligible patients (pts) included those ≥18 yrs of age who underwent complete resection of stage III cutaneous melanoma (excluding lymph node metastasis ≤1 mm or in-transit metastasis alone). 951 pts (20%/44%/36% who had stage IIIA/IIIB/IIIC, 42% ulcerated primary, and 58% macroscopic lymph node involvement) were randomized, stratified by stage and region, 1:1 to IPI 10 mg/kg (n=475) or PBO (n=476) q3w for 4 doses, then every 3 mos for up to 3 yrs until completion, disease recurrence, or unacceptable toxicity. The primary endpoint was RFS (updated here). Secondary endpoints included DMFS, OS, and safety.
Results: At 5.3 yrs median follow-up, IPI demonstrated a statistically and clinically significant improvement in OS vs. PBO (HR=0.72, i.e. 28% risk reduction of death). Similar impact for RFS and DMFS were observed.
CI: Confidence interval, *95%, **95.8% or ***95.1%; NR: not reached; †stratified by stage Among pts who started IPI (n=471) or PBO (n=474), 54.1% (IPI) and 26.2% (PBO) experienced grade 3/4 AEs, consistent with previous reports. The most common grade 3/4 immune-related adverse events (AEs) in the IPI-treated pts were gastrointestinal (16.1%), hepatic (10.8%), and endocrine (7.9%). 251 (53.3%) pts discontinued IPI due to AEs; 5 (1.1%) died due to drug-related AEs.
Conclusions: IPI as adjuvant therapy provided a clinically and statistically significant improvement in OS with a favorable benefit-risk ratio in high-risk stage III melanoma patients. These data reinforce IPI as an important treatment option for these pts.
Clinical trial identification: NCT00636168
Legal entity responsible for the study: Bristol-Myers Squibb
Funding: Bristol-Myers Squibb
Disclosure:
A.M.M. Eggermont: Advisory boards for BMS, MSD.
V. Chiarion-Sileni: Served in consulting or advisory role for Roche, BMS, GSK, MSD.
Participated in speakers' bureau for Roche, BMS, GSK. Travel, accomodations, expenses reimbursed by Roche, GSK, MSD, BMS.
J-J. Grob: Served in a consulting or advisory role for BMS, GSK, Novartis, Amgen, Merck Roche. Participated in a speakers' bureau for GSK, Roche, BMS. Conducted research project(s) funded by Roche, BMS. Travel, accomodations, & expenses paid or reimbursed by Roche.
R. Dummer: Paid honoraria from Roche, BMS, GSK, MSD, Novartis. Served in consulting or advisory role by Roche, BMS, GSK, MSD, Novartis. Conducted research project(s) funded by Roche, BMS, GSK, MSD, Novartis.
J.D. Wolchok: Hon: EMD Serono, Janssen Oncol. Consulting: BMS, Merck, MedImmune, Ziopharm, Polynoma, Polaris, Jounce, GSK. Research funding: BMS, MedImmunce, GSK, Merck. Patent: issued patent for DNA vaccines of cancer in companion animals. Expenses Reimbursed: BMS.
H. Schmidt: Consultant advisor for Bristol-Myers Squibb, GlaxoSmithKline, Merck, and Roche. He has participated in speakers’ bureau for Bristol-Myers Squibb, GlaxoSmithKline, and Roche.
O. Hamid: Reports consulting for Amgen, Novartis, Roche, BMS, Merck, Merck Serrano, Pfizer, Genentech. Reports speaker for BMS, Genentech, Novartis, outside the submitted work.
C. Robert: Honoraria: BMS, Merck, GSK, Roche, Novartis, Amgen. Consulting or Advisory Role: BMS, Merck, GSK, Roche, Novartis, Amgen.
P.A. Ascierto: Honoraria: BMS, Roche-Genentech, GSK. Research Funding: BMS, Roche-Genentech, Ventana.
C. Lebbé: Honoraria: BMS, MSD, Roche, Novartis, Amgen. Consulting or Advisory Role: Roche. Research Funding: Roche. Travel, Accomodations, Expenses: BMS, Roche (ASCO, AACR, EADO).
M. Smylie: Honoraria: BMS, Roche, GSK, Merck. Consulting or Advisory Role: BMS, Roche, GSK, Merck. Speakers' Bureau: BMS, Roche.
J.S. Weber: Stock or Other Ownership: Celldex, Altor, CCAM. Honoraria: BMS, Merck, GSK, Roche, Genentech. Consulting or Advisory Role: BMS, Merck, GSK, Roche, Genentech. Research Funding: BMS, Merck, GSK, Genentech, Roche, Monogenic.
C. Taitt: Employee: BMS. Stock or Other Ownership: BMS.
V. de Pril: Employee: BMS
G. de Schaetzen, S. Suciu: Reports grants from BMS during the conduct of the study.
All other authors have declared no conflicts of interest.
Keywords: adjuvant therapy, ipilimumab, survival, stage III melanoma