Findings from a meta-analysis of phase III randomised controlled trials presented at the ESMO Congress 2019 in Barcelona, Spain indicate that there is a gender difference in the response to immunotherapeutic agents used to treat advanced solid tumours.
Fabio Conforti, Medical Oncology of Melanoma, Sarcoma and Rare Tumours, Istituto Europeo di Oncologia in Milan, Italy and colleagues previously reported that anti-CTLA4 or anti-PD1 agents were more effective in men than women.1 Their current investigation is a meta-analysis of trials evaluating anti-PD1 and anti-PD-L1 as monotherapy and in combination with chemotherapy in male and female patients with advanced solid tumours.
Prior to conducting this meta-analysis, the investigators first reviewed all randomised controlled trials of anti-PD1 or anti-PD-L1 that were administered as monotherapy or combined with chemotherapy in patients with advanced/metastatic solid tumours.
The primary endpoint of the analysis was to assess the difference in treatment efficacy between males and females, measured in terms of difference of the overall survival (OS) and expressed as log hazard ratio (HR). The pooled OS HR and 95% confidence interval (CI) was calculated in males and females using random-effects model and the heterogeneity between the two estimates was evaluated using an interaction test.
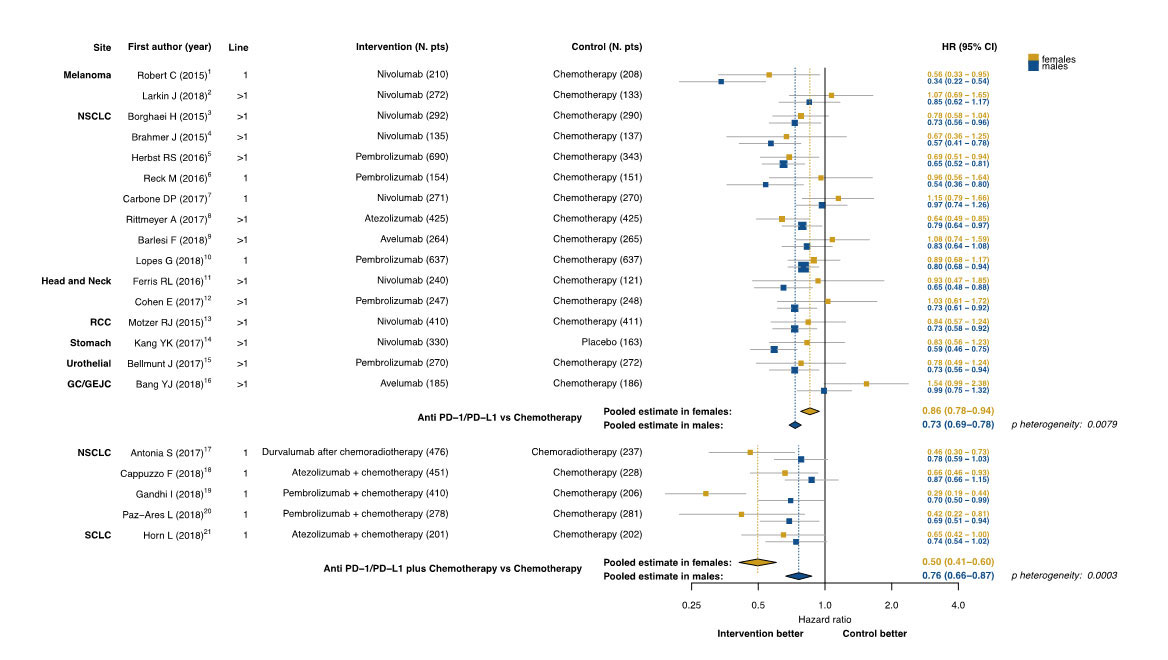
Monotherapy with an immune agent favoured males in 15 of the 16 trials evaluated
The investigators identified 16 phase III randomised controlled trials of anti-PD1 or anti-PD-L1 administered as monotherapy versus standard chemotherapy; 2 trials were carried out in patients with melanoma, 8 in non-small cell lung cancer (NSCLC), 2 in head and neck squamous cell carcinoma, 2 in gastric cancer, and 1 trial each was conducted in patients with renal and urothelial cancers.
The analysis comprised data from 9291 patients with advanced solid tumours that showed a larger benefit in males receiving immunotherapy as monotherapy. Of the 16 trials evaluated, 15 showed significantly greater OS benefit in men than women with anti-PD1 or anti-PD-L1 monotherapy; pooled-OS HR 0.73 (95% confidence interval [CI], 0.69-0.78) in men compared to HR 0.86 (95% CI, 0.78-0.94) in women (heterogeneity-p = 0.0079).
Squares indicate study-specific HRs. Size of the square is proportional to the precision of the estimate (i.e. the inverse of the variance). Horizontal lines indicate the 95% CI.
Diamonds indicates the meta-analytic pooled HRs with their corresponding 95% CIs, calculated separately in females and males for each type of immunotherapeutic strategy ( i.e. anti-PD-1/PD-L1 in monotherapy in the upper part of the figure, or combined to chemotherapy in the lower part).
The dashed vertical lines indicate the gender-specific pooled hazard ratios, and the solid vertical line indicates a hazard ratio of 1, which is the null-hypothesis value (i.e. no association between type of treatment and risk of death).
The reported p-value for heterogeneity refers to the meta-analysis of the interaction HRs (null-hypothesis: difference of immunotherapy effect between females and males is zero).
Abbreviations: NSCLC: non–small-cell lung cancer; SCLC: small cell lung cancer; RCC: renal cell carcinoma; CI confidence interval; HR. Hazard ratio.
The OS benefit with immunotherapy plus chemotherapy for lung cancer was greater in females
Further analysis of 5 phase III randomised controlled trials that evaluated anti-PD1 or anti-PD-L1 in combination with chemotherapy compared to chemotherapy in 2979 patients provided opposite results.
In the 4 NSCLC trials and one small cell lung cancer study analysed, the OS benefit in all 5 trials was significantly in favour of the women. The pooled OS HR was 0.50 (95% CI, 0.41-0.60) in women compared to 0.76 (95% CI, 0.66-0.87) in men (heterogeneity p = 0.0003).
Conclusions
The authors concluded that their analysis confirmed a large and significant interaction between the sex of the patients and the efficacy of anti-PD1 or anti-PD-L1 agents given in monotherapy or combined with chemotherapy.
They noted that the interaction was the opposite for the two immunotherapeutic strategies across the varied types of cancer studied, with women deriving more benefit from combination treatment of chemotherapy and an anti-PD1/PD-L1 agent and men benefiting more from immunotherapy administered as monotherapy.
Citation
1. Conforti F, Pala L, Bagnardi V, et al. Lancet Oncol 2018;19:737–746.
Disclosure
No external funding was disclosed.
Reference
1285P – Conforti F, Pala L. Sex-based heterogeneity of efficacy of anticancer immunotherapy.