LUGANO, Switzerland - New data presented at ESMO 2020 have shown that immunotherapy is beneficial for patients with gastric and oesophageal cancers who currently have poor survival. (1–3)
Immune therapy would be a big change in treatment, since immune checkpoint inhibitors are not yet approved for early therapy in Western countries. Three studies provide evidence, based on different patient populations and different immune checkpoint inhibitors used as first-line therapy.
CheckMate 649
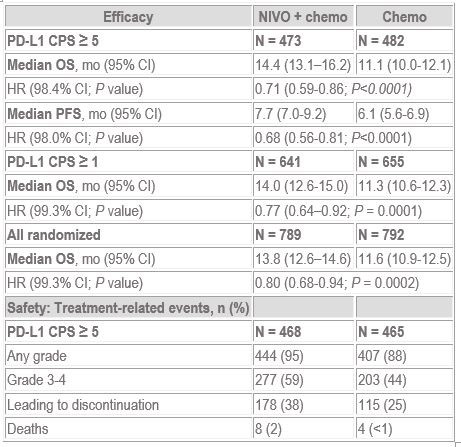
The CheckMate 649 trial (1) evaluated nivolumab plus chemotherapy versus chemotherapy alone as first-line treatment in patients with non-HER-2-positive advanced gastric cancer, gastro-oesophageal junction cancer, or oesophageal cancer – all with adenocarcinoma histology. The results show that nivolumab and chemotherapy improved overall survival and progression-free survival in patients with PD-L1 combined positive score (CPS) >5 tumours. Improvements were also observed in patients with PD-L1 CPS >1 tumours and in the overall patient population.
Additional analysis of subgroups and biomarkers (e.g. MSI-High) are planned to better characterise the efficacy benefit in patients across all CPS cutoffs.
Commenting on the new data, Prof Salah-Eddin Al-Batran, Director, Institute of Clinical Cancer Research and Director of GI Oncology, Krankenhaus Nordwest-University Cancer Centre, Frankfurt, Germany, ESMO 2020 upper GI track chair, said: “The results are clinically very relevant. Based on this trial, for patients with HER2-negative gastric adenocarcinoma, oesophageal adenocarcinoma, or gastro-oesophageal junctional adenocarcinoma with PD-L1 CPS >5 tumours, the addition of nivolumab to chemotherapy will become the standard of care for first-line treatment. The open question is the effect in patients who have a PD-L1 CPS <5.”
ATTRACTION 4
The ATTRACTION 4 trial (2) was similar to CheckMate 649 except for two important differences: it was performed only in Asian patients and the primary endpoints were designed for all-comers, rather than a specific CPS value. First-line treatment with nivolumab plus chemotherapy improved the co-primary progression-free survival endpoint, but not overall survival.
“The improvement in progression-free survival was clinically relevant and the trial strongly supports the results of CheckMate 649,” said Al-Batran. “Overall survival was not improved, possibly because all-comers were treated or because patients in Asia receive more subsequent therapies than Western populations.”
KEYNOTE 590
The KEYNOTE 590 trial (3) examined first-line chemotherapy, with or without pembrolizumab, in patients with squamous cell carcinoma of the oesophagus, adenocarcinoma of the oesophagus, or Siewert type 1 gastro-oesophageal junction adenocarcinoma. It demonstrated that pembrolizumab plus chemotherapy improved overall survival in patients with squamous cell carcinoma of the oesophagus with PD-L1 CPS >10 tumours, all squamous cell carcinomas, all patients with CPS >10, and the study population as a whole. Progression-free survival was also improved.
Most oesophageal cancer patients in the trial had squamous cell carcinoma (73%) and those with adenocarcinoma were a small subgroup. The results in the subgroup of patients with adenocarcinoma were an experimental analysis, but in the adenocarcinoma subgroup, median overall survival (OS) was 11.6 months and 9.9 months (hazard ratio [HR]=0.74), and median progression-free survival (PFS) was 6.3 months and 5.7 months (HR=0.63) in the Pembro+Chemo and Chemo group, respectively. The OS- and PFS-benefit observed in the adenocarcinoma subgroup was consistent with the benefit observed in the overall patient population.
Commenting on the findings, Al-Batran said: “I expect that KEYNOTE-590 will change practice for patients with metastatic squamous cell carcinoma or adenocarcinoma of the oesophagus who have PD-L1 CPS >10 tumours, for whom pembrolizumab added to chemotherapy will become the standard of care in the first-line.”
Al-Batran concluded: “The results of these trials offer oncologists new treatment options. In the first-line setting, there is a clear change of our standard of care, in which patients with high PD-L1 expression will be candidates for immune checkpoint inhibitors plus chemotherapy. However, more data are needed on the subgroups who benefit from the treatment (e.g. PD-L1 CPS groups and MSI).”
Notes to Editors
Please make sure to use the official name of the meeting in your reports: ESMO Virtual Congress 2020
Official Congress Hashtag: #ESMO20
Disclaimer
This press release contains information provided by the author of the highlighted abstracts and reflects the content of these abstracts. It does not necessarily reflect the views or opinions of ESMO who cannot be held responsible for the accuracy of the data. Commentators quoted in the press release are required to comply with the ESMO Declaration of Interests policy and the ESMO Code of Conduct.
References
- Abstract LBA6_PR ‘Nivolumab (NIVO) plus chemotherapy (chemo) versus chemo as first-line (1l) treatment for advanced gastric cancer/gastroesophageal junction cancer (GC/GEJC)/esophageal adenocarcinoma (EAC): First results of the CheckMate 649 study‘ will be presented by Markus Moehler during the Presidential Symposium III on Monday, 21 September, 18:30 - 20:10 CEST. Annals of Oncology, Volume 31 Supplement 4, September 2020.
- Abstract LBA7_PR ‘Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538-37) study‘ will be presented by Narikazu Boku during the Presidential Symposium III on Monday, 21 September, 18:30 - 20:10 CEST. Annals of Oncology, Volume 31 Supplement 4, September 2020.
- Abstract LBA8_PR ‘Pembrolizumab plus chemotherapy versus chemotherapy as first-line therapy in patients with advanced esophageal cancer: The phase 3 KEYNOTE-590 study’ will be presented by Ken Kato during the Presidential Symposium III on Monday, 21 September, 18:30 - 20:10 CEST. Annals of Oncology, Volume 31 Supplement 4, September 2020.
M. Moehler1, K. Shitara2, M. Garrido3, P. Salman4, L. Shen5, L. Wyrwicz6, K. Yamaguchi7, T. Skoczylas8, A. Campos Bragagnoli9, T. Liu10, M. Schenker11, P. Yanez12, M. Tehfe13, V. Poulart14, D. Cullen15, M. Lei16, K. Kondo17, M. Li18, J.A. Ajani19, Y.Y. Janjigian20
1Gastroenterology / Endosonography, Johannes-Gutenberg University Clinic, Mainz, Germany, 2Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, Kashiwa, Japan, 3Hemato-Oncology, Clinica San Carlos de Apoquindo, Pontificia Universidad Católica, Santiago Rm, Chile, 4Medical Oncology, Fundación Arturo López Pérez, Providencia, Santiago, Chile, 5Gastrointestinal Oncology, Key laboratory of Carcinogenesis and Translational Research (Ministry of Education/Beijing), Beijing Cancer Hospital, Beijing, China, 6Klinika Onkologii i Radioterapii, Narodowy Instytut Onkologii, Warszawa, Poland, 7Gastroenterological Chemotherapy, Cancer Institute Hospital of JFCR, Tokyo, Japan, 8II Klinika Chirurgii Ogólnej, Gastroenterologicznej i Nowotworów Układu Pokarmowego, Medical University of Lublin, Lublin, Poland, 9Medical Oncology, Fundacao Pio Xii Hosp Cancer De Barretos, Barretos, Sp, Brazil, 10Medical Oncology, Zhongshan Hospital Fudan University, Shanghai, China, 11Medical Oncology, SF Nectarie Oncology Center, Craiova, Romania, 12Oncology, Universidad de La Frontera, Temuco, Chile, 13Oncology Center, Centre Hospitalier de l'Universite de Montreal, Montréal, QC, Canada, 14Biostats, Bristol-Myers Squibb Company, Princeton, NJ, USA, 15Oncology Clinical Development, Bristol-Myers Squibb Company, Princeton, NJ, USA, 16Clinical Pharmacology, Bristol-Myers Squibb Company, Princeton, NJ, USA, 17Gastric and Esophageal Cancer, Bristol-Myers Squibb Company, Princeton, NJ, USA, 18Oncology clinical research, Bristol-Myers Squibb Company, Princeton, NJ, USA, 19Gastrointestinal Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX, USA, 20Gastrointestinal Oncology Service, Memorial Sloan Kettering Cancer Center and Weil Cornell Medical College, New York, NY, USA
Background: Standard 1L chemo options for advanced or metastatic HER2-negative GC/GEJC result in poor overall survival (OS; median < 1 year). CheckMate 649 is the largest randomized, global phase 3 study of programmed death (PD)-1 inhibitor-based therapies in 1L GC/GEJC/EAC. We report OS at a pre-specified interim analysis and progression-free survival (PFS) at final analysis from the NIVO + chemo vs chemo arms in patients (pts) whose tumors expressed PD-ligand 1 (L1) combined positive score (CPS) ≥ 5.
Methods: Adults with previously untreated, unresectable advanced, or metastatic GC/GEJC/EAC were enrolled, regardless of PD-L1 expression. Pts with known HER2-positive status were excluded. Pts were randomized to receive NIVO (360 mg Q3W or 240 mg Q2W) + chemo (XELOX Q3W or FOLFOX Q2W), NIVO + ipilimumab, or chemo. Dual primary endpoints for NIVO + chemo vs chemo were OS and PFS by blinded independent central review, in pts whose tumors expressed PD-L1 CPS ≥ 5.
Results: 1581 pts were concurrently randomized in nivo+chemo and chemo arms, including 955 pts (60%) with PD-L1 CPS ≥ 5. With a minimum follow-up of 12 months (mo), NIVO + chemo showed a statistically significant improvement in OS and PFS vs chemo in pts whose tumors expressed PD-L1 CPS ≥ 5 (OS, HR 0.71 [98.4% CI 0.59–0.86; P < 0.0001] and PFS, HR 0.68 [98% CI 0.56–0.81; P < 0.0001]). Statistically significant OS benefit was also observed in pts with PD-L1 CPS ≥ 1 and the all-randomized population (Table). No new safety signals were identified. Safety results are described in the Table.
Conclusions: NIVO is the first PD-1 inhibitor to demonstrate superior OS and PFS in combination with chemo vs chemo alone in previously untreated pts with advanced GC/GEJC/EAC, with a manageable safety profile. NIVO + chemo represents a potential new standard 1L treatment option for these pts.
Clinical trial identification: NCT02872116
Editorial acknowledgement: Writing and editorial assistance was provided by Tanmayi Mankame, PhD, of Parexel International, funded by Bristol-Myers Squibb Company
Legal entity responsible for the study: Bristol-Myers Squibb Company
Funding: Bristol-Myers Squibb Company
Disclosure: M. Moehler: Advisory/Consultancy, Research grant/Funding (institution), Travel/Accommodation/Expenses, Adboards: Bristol Myers Squibb. K. Shitara: Advisory/Consultancy, Research grant/Funding (institution): Astellas Pharma; Advisory/Consultancy, Research grant/Funding (institution): Eli Lilly and Company; Advisory/Consultancy: Bristol Myers Squibb; Advisory/Consultancy: Takeda Pharmaceuticals; Advisory/Consultancy: Pfizer Inc; Advisory/Consultancy: Ono Pharmaceutical; Honoraria (self), Advisory/Consultancy: Novartis; Honoraria (self), Advisory/Consultancy: AbbVie Inc; Honoraria (self): Yakult; Research grant/Funding (institution): Dainippon Sumitomo Pharma; Research grant/Funding (institution): Daiichi Sankyo; Advisory/Consultancy, Research grant/Funding (institution): Taiho Pharmaceutical; Research grant/Funding (institution): Chugai Pharma; Advisory/Consultancy, Research grant/Funding (institution): Merck Pharmaceutical; Research grant/Funding (institution): Medi Science; Advisory/Consultancy: GlaxoSmithKline. M. Garrido: Advisory/Consultancy, Research grant/Funding (institution): Bristol Myers Squibb; Advisory/Consultancy: MSD; Advisory/Consultancy, Research grant/Funding (institution): Novartis; Advisory/Consultancy: Roche. K. Yamaguchi: Advisory/Consultancy, Research grant/Funding (institution): Taiho; Advisory/Consultancy: Chugai; Research grant/Funding (institution): Sanofi; Advisory/Consultancy, Research grant/Funding (institution): Daiichi-Sankyo; Advisory/Consultancy: Lilly; Advisory/Consultancy, Research grant/Funding (institution): Ono; Advisory/Consultancy, Research grant/Funding (institution): Yakult Honsha; Advisory/Consultancy: Takeda; Advisory/Consultancy: Bristol Myers Squibb; Advisory/Consultancy: Merck Serono. M. Schenker: Research grant/Funding (self), fee for clinical research activity : Bristol Myers Squibb; Research grant/Funding (self), fee for clinical research activity : Roche; Research grant/Funding (self), fee for clinical research activity : Pfizer; Research grant/Funding (self), fee for clinical research activity : MSD; Research grant/Funding (self), fee for clinical research activity : Eli Lilly; Research grant/Funding (self), fee for clinical research activity : Novartis; Research grant/Funding (self), fee for clinical research activity : Astellas; Research grant/Funding (self), fee for clinical research activity : GSK; Research grant/Funding (self), fee for clinical research activity : Astra Zeneca; Research grant/Funding (self), fee for clinical research activity : Merck Serono; Research grant/Funding (self), fee for clinical research activity : Regeneron. M. Tehfe: Advisory/Consultancy: Bristol Myers Squibb. V. Poulart: Full/Part-time employment: Bristol Myers Squibb. D. Cullen: Travel/Accommodation/Expenses, Shareholder/Stockholder/Stock options, Full/Part-time employment: Bristol Myers Squibb. M. Lei: Shareholder/Stockholder/Stock options, Full/Part-time employment: Bristol Myers Squibb.
K. Kondo: Shareholder/Stockholder/Stock options, Full/Part-time employment: Bristol Myers Squibb.
M. Li: Full/Part-time employment: Bristol Myers Squibb. J.A. Ajani: Advisory/Consultancy, Research grant/Funding (institution): Bristol Myers Squibb. Y.Y. Janjigian: Advisory/Consultancy, Research grant/Funding (institution): Eli Lilly; Speaker Bureau/Expert testimony: ASCO; Advisory/Consultancy: Michael J. Hennessy Associates; Advisory/Consultancy: Paradigm Medical Communications, LLC; Advisory/Consultancy: Zymeworks Inc.; Advisory/Consultancy: Jounce Therapeutics; Advisory/Consultancy: Seattle Genetics; Shareholder/Stockholder/Stock options: Rgenix; Advisory/Consultancy: Astra Zeneca; Advisory/Consultancy: Daiichi Sankyo; Research grant/Funding (institution): ONO Pharma; Advisory/Consultancy, Research grant/Funding (institution): Merck & Co Inc.; Advisory/Consultancy, Research grant/Funding (institution): Bristol-Myers Squibb; Research grant/Funding (institution): Boehringer Ingelheim; Research grant/Funding (institution): Bayer; Research grant/Funding (institution): Genentech/Roche; Advisory/Consultancy: Merck Serono; Advisory/Consultancy: Pfizer; Advisory/Consultancy: Imugene. All other authors have declared no conflicts of interest.
N. Boku1, M.H. Ryu2, D-Y. Oh3, S.C. Oh4, H.C. Chung5, K-W. Lee6, T. Omori7, K. Shitara8, S. Sakuramoto9, I.J. Chung10, K. Yamaguchi11, K. Kato12, S.J. Sym13, S. Kadowaki14, K. Tsuji15, J-S. Chen16, L-Y. Bai17, L-T. Chen18, Y-K. Kang19
1Department of Gastrointestinal Medical Oncology, National Cancer Center Hospital, Tokyo, Japan, 2Oncology Dept, Asan Medical Center - University of Ulsan College of Medicine, Seoul, Korea, Republic of, 3Internal Medicine, Seoul National University Hospital, Seoul, Korea, Republic of, 4Dept of Medical Oncology, Korea University Guro Hospital, Seoul, Korea, Republic of, 5Medical Oncology Dept, Yonsei University, Seoul, Korea, Republic of, 6Medical Oncology, Seoul National University Bundang Hospital, Seoul National University College of Medicine, Seongnam, Gyeonggi-do, Korea, Republic of, 7Gastroenterological Surgery, Osaka International Cancer Institute, Osaka, Japan, 8Department of Gastrointestinal Oncology, National Cancer Center Hospital East, Kashiwa, Chiba, Japan, 9Department of Gastroenterology, Saitama Medical University, International Cancer Center, Saitama, Japan, 10Internal medicine/Hemato-oncology, Chonnam National University Hwasun Hospital, Hwasun, Korea, Republic of, 11Department of Gastroenterological Chemotherapy, Cancer Institute Hospital of JFCR, Tokyo, Japan, 12Department of Gastrointestinal Medical Oncology, National Cancer Center Research Institute - Tsukiji Campus, Chuo-ku, Japan, 13Internal Medicine, Division of Medical Oncology, Gachon University Gil Medical Center, Incheon, Korea, Republic of, 14Department of Clinical Oncology, Aichi Cancer Center Hospital, Nagoya, Japan, 15Gastroenterology, Ishikawa Prefectural Central Hospital, Ishikawa, Japan, 16Division of Hematology-Oncology, Chang Gung Memorial Hospital at Linkou, Taoyuan City, Taiwan, 17Division of Hematology and Oncology, Department of Internal Medicine, China Medical University Hospital, Taichung, Taiwan, 18National Cheng Kung University Hospital, National Institute of Cancer Research, Tainan, Taiwan, 19Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Songpa-gu, Korea, Republic of
Background: Nivolumab has a survival benefit for heavily pretreated patients with advanced or recurrent G/GEJ cancer. ATTRACTION-4 is a randomized, multicenter, phase 2/3 study to evaluate the efficacy and safety of nivolumab plus chemotherapy vs. chemotherapy as first-line treatment in patients with HER2-negative, advanced or recurrent G/GEJ cancer. Here we report the results of the double-blind phase 3 part.
Methods: Patients were randomized 1:1 to receive nivolumab plus chemotherapy (N+C, S-1 plus oxaliplatin or capecitabine plus oxaliplatin) or placebo plus chemotherapy (C). Nivolumab or placebo was intravenously administered every 3 weeks until disease progression or unacceptable toxicity. Tumor assessment was performed every 6 weeks through week 54, then repeated every 12 weeks. The co-primary endpoints were centrally-assessed PFS and OS, and it was prespecified that the primary objective is deemed to be achieved if at least one of the null hypotheses of the primary endpoints is rejected.
Results: A total of 724 Asian patients were randomized to N+C (n=362) or C (n=362) between Mar 7, 2017, and May 10, 2018. At the interim analysis primary for PFS with the median follow-up period of 11.6 mo, PFS was significantly improved in N+C vs. C (HR 0.68; 98.51% CI 0.51-0.90; p=0.0007; median PFS, 10.5 vs. 8.3 mo), meeting the primary endpoint. At the final analysis primary for OS with the median follow-up period of 26.6 mo, there was no statistically significant difference (HR 0.90; 95% CI 0.75-1.08; p=0.257; median OS, 17.5 vs. 17.2 mo), while PFS was continuously longer in N+C than in C. ORR was higher in N+C than in C (57.5 vs. 47.8%; p=0.0088). The incidences of grade 3 to 5 treatment-related adverse events were 57.9% in N+C and 49.2% in C.
Conclusions: PFS was significantly improved in N+C vs. C, achieving the primary objective. The combination of nivolumab and chemotherapy, which demonstrated clinically meaningful efficacy in PFS and ORR with a manageable safety profile but not statistically significant improvement in OS, can be considered a new first-line treatment option in advanced or recurrent G/GEJ cancer.
Clinical trial identification: Trial protocol number: NCT02746796 Study Start Date: March 2016
Legal entity responsible for the study: Ono Pharmaceutical CO., Ltd.
Funding: Ono Pharmaceutical Co., Ltd. Bristol-Myers Squibb
Disclosure: N. Boku: Honoraria (self): ONO; Honoraria (self), Research grant/Funding (institution): BMS; Honoraria (self), Research grant/Funding (institution): Taiho. M.H. Ryu: Honoraria (self), Advisory/Consultancy: ONO; Honoraria (self), Advisory/Consultancy: BMS; Honoraria (self), Advisory/Consultancy: MSD; Honoraria (self), Advisory/Consultancy: Eli Lilly; Honoraria (self), Advisory/Consultancy: Taiho; Honoraria (self), Advisory/Consultancy: Novartis; Honoraria (self), Advisory/Consultancy: Daehwa. D-Y. Oh: Advisory/Consultancy, Research grant/Funding (self): AstraZeneca; Advisory/Consultancy, Research grant/Funding (self): Novartis; Advisory/Consultancy: Genentech; Advisory/Consultancy: Merck Serono; Advisory/Consultancy: Bayer; Advisory/Consultancy: Taiho; Advisory/Consultancy: ASLAN; Advisory/Consultancy: Halozyme; Advisory/Consultancy: Zymeworks; Advisory/Consultancy: Celgene; Research grant/Funding (self): Array; Research grant/Funding (self): Eli Lilly; Advisory/Consultancy: Roche. H.C. Chung: Honoraria (self), Advisory/Consultancy, Research grant/Funding (institution): Merck-Serono; Honoraria (self), Advisory/Consultancy, Research grant/Funding (institution): Eli Lilly; Honoraria (self): Foundation Medicine; Advisory/Consultancy, Research grant/Funding (institution): Taiho; Advisory/Consultancy: Celltrion; Advisory/Consultancy, Research grant/Funding (institution): MSD; Advisory/Consultancy, Research grant/Funding (institution): BMS; Advisory/Consultancy: Gloria; Advisory/Consultancy, Research grant/Funding (institution): Beigene; Advisory/Consultancy, Research grant/Funding (institution): Amgen; Advisory/Consultancy: Zymework; Research grant/Funding (institution): GSK; Research grant/Funding (institution): Ono pharmaceutical. K-W. Lee: Honoraria (self): BMS; Honoraria (self): Eli Lilly; Honoraria (self): Genexine; Research grant/Funding (institution): Macrogenics; Research grant/Funding (institution): MSD; Research grant/Funding (institution): Ono pharmaceutical; Research grant/Funding (institution): Green Cross Corp.; Research grant/Funding (institution): ASLAN pharmaceuticals; Research grant/Funding (institution): AstraZeneca/MedImmune; Research grant/Funding (institution): Five Prime Therapeutics; Research grant/Funding (institution): LSK BioPharma; Research grant/Funding (institution): Merck KGaA; Research grant/Funding (institution): Array BioPharma; Research grant/Funding (institution): Pharmacyclics; Research grant/Funding (institution): Pfizer; Research grant/Funding (institution): ALX Oncology; Research grant/Funding (institution): Zymeworks; Research grant/Funding (institution): BeiGene; Research grant/Funding (institution): Daiichi Sankyo; Research grant/Funding (institution): Taiho Pharmaceutical. K. Shitara: Honoraria (self), Advisory/Consultancy: Novartis; Honoraria (self), Advisory/Consultancy: AbbVie; Honoraria (self): Yakult; Advisory/Consultancy, Research grant/Funding (institution): Astellas; Advisory/Consultancy, Research grant/Funding (institution): Eli Lilly; Advisory/Consultancy: BMS; Advisory/Consultancy: Takeda; Advisory/Consultancy: Pfizer; Advisory/Consultancy, Research grant/Funding (institution): Ono pharmaceutical; Advisory/Consultancy, Research grant/Funding (institution): MSD; Advisory/Consultancy, Research grant/Funding (institution): Taiho; Advisory/Consultancy: GSK; Research grant/Funding (institution): Sumitomo Dainippon Pharma; Research grant/Funding (institution): Daiichi Sankyo; Research grant/Funding (institution): Chugai; Research grant/Funding (institution): Medi Science.
S. Sakuramoto: Research grant/Funding (institution): Ono pharmaceutical; Research grant/Funding (institution): Taiho; Research grant/Funding (institution): Kaken pharmaceutical; Research grant/Funding (institution): Chugai.
K. Yamaguchi: Honoraria (institution), Speaker Bureau/Expert testimony: Daiichi Sankyo; Honoraria (institution), Speaker Bureau/Expert testimony: Taiho; Honoraria (institution), Speaker Bureau/Expert testimony: Chugai; Speaker Bureau/Expert testimony: BMS; Honoraria (institution), Speaker Bureau/Expert testimony: ONO pharmaceutical; Speaker Bureau/Expert testimony: Takeda; Honoraria (institution), Speaker Bureau/Expert testimony: Eli Lilly; Honoraria (institution), Speaker Bureau/Expert testimony: Sanofi; Honoraria (institution): MSD oncology; Honoraria (institution): Sumitomo Dainippon Pharma; Honoraria (institution): Gilead Sciences; Honoraria (institution): Boehringer Ingelheim; Honoraria (institution): Eisai; Honoraria (institution): Yakult Honsya. K. Kato: Advisory/Consultancy, Speaker Bureau/Expert testimony, Research grant/Funding (institution): Ono pharmaceutical; Advisory/Consultancy, Research grant/Funding (institution): Beigene; Advisory/Consultancy, Research grant/Funding (institution): MSD; Speaker Bureau/Expert testimony: Taiho; Speaker Bureau/Expert testimony: Eli Lilly; Speaker Bureau/Expert testimony, Research grant/Funding (institution): BMS; Research grant/Funding (institution): Shionogi; Research grant/Funding (institution): Merck Bio; Research grant/Funding (institution): Chugai. S. Kadowaki: Honoraria (self), Research grant/Funding (institution): Eli Lilly; Honoraria (self), Research grant/Funding (institution): Taiho; Honoraria (self), Research grant/Funding (institution): Ono pharmaceutical; Honoraria (self), Research grant/Funding (institution): BMS; Honoraria (self): Yakult Honsya; Honoraria (self): Chugai; Honoraria (self): Bayer; Honoraria (self): Merck; Research grant/Funding (institution): MSD. J-S. Chen: Honoraria (self), Advisory/Consultancy: Ono pharmaceutical; Honoraria (self): TTY Biopharma; Honoraria (self): MSD oncology; Honoraria (self): Medimmune; Honoraria (self): Merck KGaA; Honoraria (self): Roche; Honoraria (self): AstraZeneca.
L-Y. Bai: Honoraria (self): AbbVie; Honoraria (self): Bayer; Honoraria (self): BMS; Honoraria (self): Johnson & Johnson; Honoraria (self): GSK; Honoraria (self): Eli Lilly; Honoraria (self): MSD; Honoraria (self): Novartis; Honoraria (self): Ono pharmaceutical; Honoraria (self): Pfizer; Honoraria (self): PharmaEngine; Honoraria (self): Roche; Honoraria (self): SynCore Biotechnology; Honoraria (self): Takeda; Honoraria (self): TTY Biopharm. L-T. Chen: Honoraria (self): Ono pharmaceutical; Honoraria (self): Eli Lilly; Honoraria (self): MSD; Honoraria (self), Advisory/Consultancy: PharmaEngine; Honoraria (self), Research grant/Funding (institution): TTY Biopharm; Honoraria (self), Research grant/Funding (institution): SyncorePharm; Honoraria (self), Research grant/Funding (institution): Novartis; Honoraria (self): AstraZeneca; Honoraria (self): Ipsen; Leadership role: National Institute of Cancer Research, Taiwan; Research grant/Funding (institution): Merck Serono; Research grant/Funding (institution): Polaris; Research grant/Funding (institution): Pfizer; Research grant/Funding (institution): BMS; Licensing/Royalties: ENO-1 mAb from HuniLife; Full/Part-time employment: National Health Research Institutes, Taiwan; Officer/Board of Directors: SinoPharm Taiwan, Ltd. Y-K. Kang: Advisory/Consultancy: ALX Oncology; Advisory/Consultancy: BMS; Advisory/Consultancy: Amgen; Advisory/Consultancy: Daehwa; Advisory/Consultancy: Macrogenics; Advisory/Consultancy: Novartis; Advisory/Consultancy: Surface Oncology; Advisory/Consultancy: Zymeworks.
All other authors have declared no conflicts of interest.
K. Kato1, J-M. Sun2, M.A. Shah3, P.C. Enzinger4, A. Adenis5, T. Doi6, T. Kojima7, J-P. Metges8, Z. Li9, S-B. Kim10, B.C. Chul Cho11, W. Mansoor12, S-H. Li13, P. Sunpaweravong14, M.A. Maqueda15, E. Goekkurt16, Q. Liu17, S. Shah17, P. Bhagia17, L. Shen18
1Gastrointestinal Medical Oncology, National Cancer Center Hospital, Tokyo, Japan, 2Medicine, Samsung Medical Center, Sungkyunkwan University, Seoul, Korea, Republic of, 3Medical Oncology/Solid Tumor Program, Weill Cornell Medical College, New York, NY, USA, 4Medical Oncology, Dana Farber Cancer Institute, Boston, MA, USA, 5Medical Oncology, IRCM, Inserm, Université Montpellier, ICM, Montpellier, CEDEX, France, 6Experimental Therapeutics, National Cancer Center Hospital East, Chiba, Chiba, Japan, 7Gastroenterology and Gastrointestinal Oncology, National Cancer Center Hospital East, Kashiwa, Chiba, Japan, 8Cancer Institute, CHU Brest – Institut de Cancerologie et d’Hematologie, Brest, Cedex 2, France, 9Thoracic & Esophageal Surgery, Shanghai Chest Hospital, Esophageal Disease Center,, Shanghai, China, 10Oncology, Asan Medical Center, Seoul, Korea, Republic of, 11Yonsei Cancer Center, Severance Hospital, Yonsei University Health System, Seoul, Korea, Republic of, 12Medical Oncology, Christie Hospital NHS Trust, Manchester, UK, 13Oncology and Hematology, Kaohsiung Chang Gung Memorial Hospital and Chang Gung University College of Medicine, Kaohsiung, Taiwan, 14Medicine, Prince of Songkla University Hospital, Songkhla, Thailand, 15Medical Oncology, Vall d’Hebron Institute of Oncology, Barcelona, Spain, 16Hematology-Oncology, Hematology Oncology Practice Eppendorf, and University Cancer Center Hamburg, Hamburg, Germany, 17Medical Oncology, Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA, 18Gastrointestinal Oncology, Peking University Cancer Hospital & Institute, Beijing, China
Background: KEYNOTE-590 (NCT03189719) is a randomized, international, double-blind study of 1L pembrolizumab (pembro) + chemotherapy (chemo) vs chemo alone in patients (pts) with locally advanced/unresectable or metastatic adenocarcinoma or esophageal squamous cell carcinoma (ESCC) or Siewert type 1 esophagogastric junction adenocarcinoma (EGJ).
Methods: Eligible pts were randomized 1:1 to pembro 200 mg or placebo Q3W for up to 2 yr + chemo (cisplatin 80 mg/m2 Q3W [d1; 6 doses] + 5-FU 800 mg/m2 on d1-5 Q3W). Randomization was stratified by Asia vs Rest of World, adenocarcinoma vs ESCC, and ECOG PS 0 vs 1. Treatment continued until progression, unacceptable toxicity, or withdrawal, or 2 yr. No crossover was permitted. Primary end points were OS in pts with ESCC PD-L1 combined positive score (CPS) ≥10 tumors, and OS and PFS (RECIST v1.1 ; by investigator) in ESCC, PD-L1 CPS ≥10, and all pts. The secondary end point was ORR (RECIST v1.1; by investigator) in all pts. Data cutoff for interim OS/final PFS analysis was July 2, 2020.
Results: At data cutoff, 749 pts (83% male, 73% ESCC) were randomized (373 pembro + chemo; 376 chemo). Median follow-up was 10.8 mo. Pembro + chemo vs chemo was superior for OS in pts with ESCC CPS ≥10 (median 13.9 vs 8.8 mo; HR 0.57; 95% CI, 0.43-0.75; P < 0.0001), ESCC (median 12.6 vs 9.8 mo; HR 0.72; 95% CI, 0.60-0.88; P = 0.0006), CPS ≥10 (median 13.5 vs 9.4 mo; HR 0.62; 95% CI, 0.49-0.78; P < 0.0001), and all pts (median 12.4 vs 9.8 mo; HR, 0.73, 95% CI, 0.62-0.86; P < 0.0001). PFS was superior with pembro + chemo vs chemo in ESCC (median 6.3 vs 5.8 mo; HR 0.65; 95% CI, 0.54-0.78; P < 0.0001), CPS ≥10 (median 7.5 vs 5.5 mo; HR 0.51; 95% CI, 0.41-0.65; P < 0.0001), and all pts (median 6.3 vs 5.8 mo; HR 0.65; 95% CI, 0.55-0.76; P < 0.0001). Confirmed ORR was 45.0% vs 29.3% (P < 0.0001) in all pts, with median DOR of 8.3 vs 6.0 mo. Grade 3-5 drug-related AE rates were 72% vs 68%. Discontinuation rates from drug-related AEs were 19% vs 12%.
Conclusions: Pembro + chemo provided superior OS, PFS, and ORR vs chemo, with a manageable safety profile in pts with untreated, advanced esophageal and EGJ cancer. These data demonstrate that 1L pembro + chemo is a new standard of care in this pt population.
Clinical trial identification: Clinicaltrials.gov identifier NCT03189719
Editorial acknowledgement: Luana Atherly-Henderson of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA
Legal entity responsible for the study: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA
Funding: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA
Disclosure: K. Kato: Advisory/Consultancy, Research grant/Funding (institution): BMS; Advisory/Consultancy, Research grant/Funding (institution): MSD; Advisory/Consultancy, Research grant/Funding (institution): ONO; Advisory/Consultancy, Research grant/Funding (institution): Beigene; Speaker Bureau/Expert testimony: Taiho; Speaker Bureau/Expert testimony: Eli Lilly; Research grant/Funding (institution): Shionogi; Research grant/Funding (institution): Merck Bio; Research grant/Funding (institution): Chugai. J-M. Sun: Research grant/Funding (institution): AstraZeneca; Research grant/Funding (institution): Ono; Research grant/Funding (institution): MSD. M.A. Shah: Advisory/Consultancy: Daiichi; Advisory/Consultancy, Research grant/Funding (institution): Merck Sharp & Dohme Corp.; Research grant/Funding (institution): Boston Biomedical; Research grant/Funding (institution): Roche; Research grant/Funding (institution): Oncolys. P.C. Enzinger: Honoraria (self), Advisory/Consultancy: Astellas; Honoraria (self), Advisory/Consultancy: AstraZeneca; Honoraria (self), Advisory/Consultancy: Celgene; Honoraria (self), Advisory/Consultancy: Daiichi-Sankyo; Honoraria (self), Advisory/Consultancy: Five-Prime; Honoraria (self), Advisory/Consultancy: Lilly; Honoraria (self), Advisory/Consultancy: Loxo; Honoraria (self), Advisory/Consultancy: Merck Sharp & Dohme Corp.; Honoraria (self), Advisory/Consultancy: Taiho; Honoraria (self), Advisory/Consultancy: Takeda; Honoraria (self), Advisory/Consultancy: Zymeworks. A. Adenis: Honoraria (self), Advisory/Consultancy, Research grant/Funding (institution), Travel/Accommodation/Expenses: Bayer; Honoraria (self), Advisory/Consultancy, Research grant/Funding (institution), Travel/Accommodation/Expenses: Bristol Myers Squibb; Honoraria (self), Research grant/Funding (institution): Sanofi; Advisory/Consultancy: Servier; Research grant/Funding (institution), Travel/Accommodation/Expenses: Merck Sharp & Dohme Corp.; Research grant/Funding (institution): Pfizer. T. Doi: Honoraria (self), Research grant/Funding (institution): Bristol Myers Squibb; Honoraria (self): Ono Pharmaceutical; Honoraria (self), Advisory/Consultancy, Research grant/Funding (institution): Abbvie; Honoraria (self): Astellas Pharma; Honoraria (self): Oncolys BioPharma; Honoraria (self), Advisory/Consultancy, Research grant/Funding (institution): Taiho Pharmaceutical; Honoraria (self): Otsuka; Advisory/Consultancy, Research grant/Funding (institution): MSD; Advisory/Consultancy, Research grant/Funding (institution): Daiichi Sankyo; Advisory/Consultancy: Amgen; Advisory/Consultancy, Research grant/Funding (institution): Sumitomo Dainippon; Advisory/Consultancy: Takeda; Advisory/Consultancy, Research grant/Funding (institution): Novartis; Advisory/Consultancy: Bayer; Advisory/Consultancy, Research grant/Funding (institution): Boehringer Ingelheim; Advisory/Consultancy: Rakuten Medical; Research grant/Funding (institution): Merck Serono; Research grant/Funding (institution): Pfizer; Research grant/Funding (institution): Lilly; Research grant/Funding (institution): Kyowa Hakko Kirin; Research grant/Funding (institution): Eisai; Research grant/Funding (institution): IQVIA.
T. Kojima: Honoraria (self), Research grant/Funding (institution): Ono Pharmaceutical; Honoraria (self): Oncolys BioPharma; Advisory/Consultancy: Astellas Pharma; Advisory/Consultancy: Bristol Myers Squibb; Advisory/Consultancy, Research grant/Funding (institution): MSD; Advisory/Consultancy, Research grant/Funding (institution): Ono Pharmaceutical; Advisory/Consultancy: Merck; Research grant/Funding (institution): Astellas Amgen BioPharma; Research grant/Funding (institution): Taiho Pharmaceutical; Research grant/Funding (institution): Shionogi. J-P. Metges: Honoraria (self): MSD; Honoraria (self): Bayer; Honoraria (self): Bristol Myers Squibb.
S-B. Kim: Advisory/Consultancy, Research grant/Funding (self): Novartis; Research grant/Funding (self): Sanofi-Aventis; Research grant/Funding (self): DongKook Pharma Co.; Advisory/Consultancy: AstraZeneca; Advisory/Consultancy: Lilly; Advisory/Consultancy: Enzychem; Advisory/Consultancy: Dae Hwa Pharmaceutical Co. Ltd; Advisory/Consultancy: ISU Abxis; Advisory/Consultancy: Daiichi-Sankyo. B.C. Chul Cho: Advisory/Consultancy, Research grant/Funding (institution): Novartis; Advisory/Consultancy, Research grant/Funding (institution): AstraZeneca; Advisory/Consultancy: Boehringer Ingelheim; Advisory/Consultancy: Roche; Advisory/Consultancy: Bristol Myers Squibb; Advisory/Consultancy, Research grant/Funding (institution): Ono; Advisory/Consultancy, Research grant/Funding (institution): Yuhan; Advisory/Consultancy: Pfizer; Advisory/Consultancy, Research grant/Funding (institution): Eli Lilly; Advisory/Consultancy, Research grant/Funding (institution): Janssen; Advisory/Consultancy: Medpacto; Advisory/Consultancy, Research grant/Funding (institution): Blueprint Medicines; Advisory/Consultancy, Shareholder/Stockholder/Stock options: KANAPH Therapeutic Inc.; Advisory/Consultancy, Shareholder/Stockholder/Stock options: Brigebio Therapeutics; Advisory/Consultancy, Shareholder/Stockholder/Stock options: Cyrus Therapeutics; Advisory/Consultancy: Guardant Health; Shareholder/Stockholder/Stock options: TheraCanVac Inc; Shareholder/Stockholder/Stock options, Officer/Board of Directors: Gencurix Inc; Research grant/Funding (institution), Shareholder/Stockholder/Stock options, Officer/Board of Directors: Interpark Bio Convergence Corp; Research grant/Funding (institution): Bayer; Research grant/Funding (institution): MOGAM Institute; Research grant/Funding (institution): Dong-A ST; Research grant/Funding (institution), Licensing/Royalties: Champions Oncology; Research grant/Funding (institution): Dizal Pharma; Advisory/Consultancy, Research grant/Funding (institution): MSD; Research grant/Funding (institution): Abbvie; Research grant/Funding (institution): Medpacto; Research grant/Funding (institution): GIInnovation; Leadership role, founder: DAAN Biotherapeutics; Advisory/Consultancy: Takeda. S-H. Li: Research grant/Funding (institution): MSD.
M.A. Maqueda: Honoraria (self), Advisory/Consultancy, Travel/Accommodation/Expenses: Servier; Honoraria (self), Advisory/Consultancy: Bristol Myers Squibb; Honoraria (self), Advisory/Consultancy: MSD; Honoraria (self), Advisory/Consultancy, Travel/Accommodation/Expenses: Lilly; Honoraria (self), Travel/Accommodation/Expenses: Roche; Honoraria (self), Travel/Accommodation/Expenses: Amgen. E. Goekkurt: Honoraria (self): MSD; Honoraria (self): Bristol Myers Squibb; Honoraria (self): Servier. Q. Liu: Shareholder/Stockholder/Stock options, Full/Part-time employment: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.. S. Shah: Shareholder/Stockholder/Stock options, Full/Part-time employment: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.. P. Bhagia: Shareholder/Stockholder/Stock options, Full/Part-time employment: Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc.. L. Shen: Advisory/Consultancy: Merck Sharp & Dohme Corp.; Advisory/Consultancy: Harbour; Research grant/Funding (self): Boehringer Ingelheim; Research grant/Funding (self): Beijing Xiantong Biomedical Technology; Research grant/Funding (self): Qilu Pharmaceutical; Research grant/Funding (self): Zaiding Pharmaceutical; Research grant/Funding (self): Jacobio Pharmaceuticals; Research grant/Funding (self): Beihai Kangcheng(Beijing)Medical Technology Co.,Ltd. All other authors have declared no conflicts of interest.