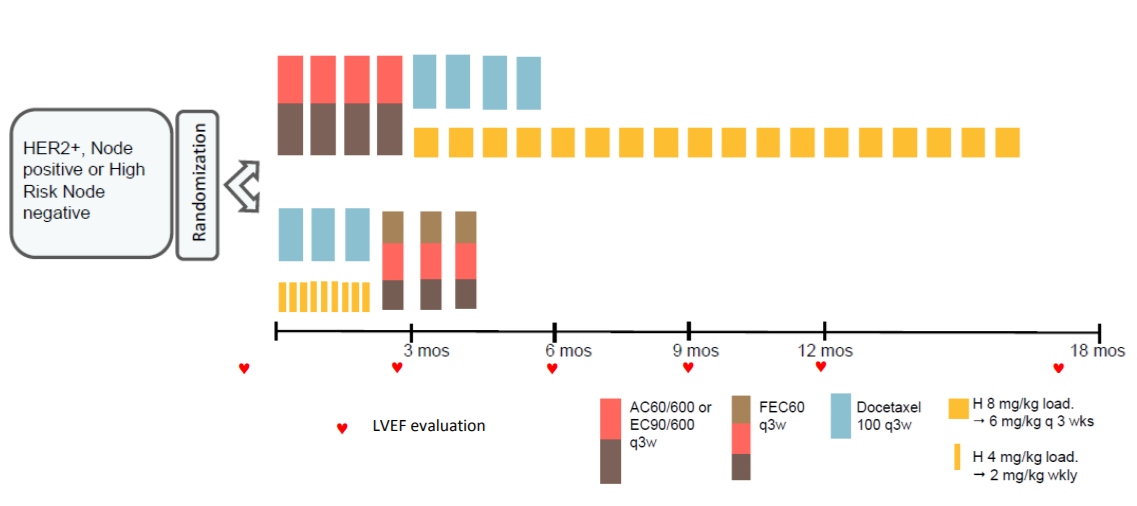
MUNICH, Germany - Women with HER2-positive early breast cancer with small tumours have similar disease-free survival and lower risk of cardiac toxicity with a nine-week course of adjuvant trastuzumab compared to those treated for one year, according to a subgroup analysis of the Short-HER trial reported at ESMO 2018 Congress in Munich (1).
A second study showed that a six-month course of adjuvant trastuzumab was cost-effective compared to 12 months, with an average cost saving of nearly £10,000 (Euros 11,300) per patient (2).
Current guidelines recommend one year of anti-HER2 antibody therapy as part of standard adjuvant treatment for HER2-positive early breast cancer patients (3) based on the duration of treatment used in pivotal registration trials. There is interest in whether a shorter course of trastuzumab could potentially achieve similar efficacy with lower risk of side-effects and costs.
The Short-HER trial randomised 1254 HER2-positive early breast cancer patients to either nine weeks or one year’s treatment with trastuzumab, with both groups also receiving chemotherapy. Results after a median of six years’ follow-up showed the short course did not achieve non-inferiority but was associated with a reduction in the rate of severe cardiac toxicity (4).
The researchers have now analysed whether there are subgroups of patients where a shorter course of trastuzumab may be non-inferior to a longer course. Multivariate analysis showed that pathologic tumour size (pT) and nodal status (N) were independent prognostic factors for disease-free survival (DFS).
They identified three prognostic groups:
- low risk (pathologic tumour size [pT] < 2cm and N0), accounting for 37.5% of patients
- intermediate risk (pT < 2cm and any N category), accounting for 51.9%
- high risk (pT > 2cm and N4+), accounting for 10.5% of the patient population.
Results showed that patients with low and intermediate risk had similar five-year DFS with a nine-week course of trastuzumab (88%) as with one year (89%; hazard ratio 1.02, 95% confidence interval [CI] 0.78-1.33) but their risk of cardiac events was nearly three times lower (4.5% vs 12.8%, relative risk 2.88 95% CI 1.85-4.47). Women at low and intermediate risk of relapse accounted for 89% of patients in the study.
“The study was underpowered because of difficulties in recruiting patients in a reasonable time so non-inferiority could not be claimed based on the results,” said lead author Pierfranco Conte, Professor of Oncology at the University of Padua and Director of the Division of Medical Oncology at the Instituto Oncologico Veneto, Padua, Italy. He added: “Based on our data, one year trastuzumab remains the standard treatment for women with HER2-positive early breast cancer”.
However, Conte added: “Physicians can stop trastuzumab before one year in patients who develop a cardiac event during treatment without compromising efficacy and can consider shorter-duration trastuzumab treatment in patients at risk of cardiac toxicity and a low or intermediate risk of breast cancer relapse.” He suggested that consideration of shorter-duration trastuzumab may also facilitate access to patients who cannot afford a longer course.
Commenting on the study for ESMO, Prof. Nadia Harbeck, Head of the Breast Center, University of Munich, said: “The results of this analysis showed that patients with a high tumour load definitely derive substantial benefit from longer duration trastuzumab.”
She added: “The results may impact on clinical decision-making, although it is an exploratory analysis of a negative trial so does not meet the criteria to be practice changing. I think it will influence clinicians and patients in that if patients cannot complete one year of trastuzumab, those patients with low tumour burden can feel reassured that they have not lost out on efficacy.”
Six-month course of trastuzumab saves nearly £10,000 per patient
A second study showed that a six-month course of adjuvant trastuzumab was cost-effective compared to 12 months, giving an average cost saving of nearly £10 000 (Euros 11,300) per patient with no evidence of detriment to quality of life (2).
Researchers analysed the cost-effectiveness of a six-month course of adjuvant trastuzumab compared to the standard 12-month course in patients with HER2 positive early breast cancer taking part in the PERSEPHONE trial. This is the largest phase 3 randomised trial to compare six months with 12 months of trastuzumab and demonstrated non-inferiority of reduced-duration trastuzumab (5).
The new study analysed health service activity and costs in addition to quality of life in 4009 patients who were disease free at six months; 250 were excluded due to lack of data. In a landmark analysis six months into treatment the researchers estimated the cost-effectiveness of six months of trastuzumab compared to a 12-month course of trastuzumab from the perspective of the health and social care sector over two years of follow-up.
Results showed the average costs for an individual patient treated with trastuzumab for six months were £2,538.64 (95% confidence interval [CI] £2,383.38-£2,700.72) compared to average costs per patient of £12,333.83 (95% CI £12,098.58-£12,562.27) in those treated with trastuzumab for 12 months.
Treating for six months gave an average cost saving of £9,793.25 (95% CI £9,515.86-£10,071.64) per patient. Trastuzumab treatment and administration accounted for the vast majority of this cost saving, with the remainder arising from cardiac assessment and treatment costs and inpatient days.
The average Quality Adjusted Life Years (QALYs) for an individual in the 6-month arm and 12-month arm were 1.146 (95% CI: 1.131 – 1.161) and 1.128 (95% CI: 1.113 – 1.144), respectively, giving an average QALY difference of 0.018 (95% CI: – 0.003 – 0.039). Thus the 6-month treatment arm dominated, with a probability of being cost-effective of 100%.
“A six-month duration of adjuvant trastuzumab with chemotherapy was found to be cost-effective compared with 12 months, which is currently the standard of care,” said lead author Claire Hulme, Professor of Health Economics at the Academic Unit of Health Economics, University of Leeds, Leeds, UK.
She added: “The results, alongside the clinical effectiveness results demonstrating non-inferiority, are the first steps in the safe reduction of treatment for many women with HER-2 positive breast cancer. They present an opportunity for significant cost savings for health service providers.”
Hulme acknowledged that a limitation of the study was that it was a landmark study, which meant the researchers looked only at a specific timepoint from six months into trastuzumab treatment. She said the research group intends to carry out further sensitivity analysis and to assess the financial costs of treatment to patients rather than only from the perspective of health service providers.
Harbeck commented: “Showing that it was more economical to treat with six months of trastuzumab versus 12 months is an important contribution for global access to treatment.” But she added: “The subgroup analysis of PERSEPHONE could not exclude a benefit of one year trastuzumab in clinically relevant subgroups, so one year remains the standard duration. And we now have biosimilars, which could also help to increase access to treatment in countries where there is no general access.”
Looking to the future, she said: “We should not do these trials assuming ‘one size fits all’ for the whole population but we should take into account patients’ individual responses to neoadjuvant anti-HER2 therapy and ask the question whether patients who have sufficient response may forgo further therapy.” She noted that recent research has also shown that high-risk patients benefit from two HER-2 antibodies, such as trastuzumab and pertuzumab, more than one.
Notes to Editors
Please make sure to use the official name of the meeting in your reports: ESMO 2018 Congress
Official Congress hashtag: #ESMO18
References
- Abstract 191PD_PR ‘9 weeks versus 1 year adjuvant trastuzumab for HER2+ early breast cancer: subgroup analysis of the ShortHER trial allows to identify patients for whom a shorter trastuzumab administration may have a favourable risk/benefit ratio’ will be presented by Pierfranco Conte during the Poster Discussion Session on Saturday 20 October, 15:00 to 16:15 (CEST) in Room 15 - Hall A1. Annals of Oncology, Volume 29 Supplement 8 October 2018
- Abstract LBA12_PR ‘PERSEPHONE: 6 versus 12 months (M) of adjuvant trastuzumab in patients (PTS) with HER2 positive early breast cancer (EBC): cost effectiveness analysis results’ will be presented by Claire Hulme during the Proffered Paper Session on Friday 19 October, 16:00 to 17:30 (CEST) in Room 13 – ICM. Annals of Oncology, Volume 29 Supplement 8 October 2018
- Senkus E, Kyriades S, Ohno S et al. Primary breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2015; 26: v8-v30.
- Conte PF, Bisagni G, Frassoldati A et al. 9 weeks vs 1 year adjuvant trastuzumab in combination with chemotherapy: Results of the phase III multicentric Italian study Short-HER. J Clin Oncol 2017; 35 suppl; abstract 501.
- Early HM, Hiller L, Vallier A-L et al. PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients with HER2 positive early breast cancer: randomised phase 3 non-inferiority trial with definitive 4-year disease-free survival results. Journal of Clinical Oncology 36, no. 15_suppl (May 20 2018) 506-506.
About the European Society for Medical Oncology (ESMO)
ESMO is the leading professional organisation for medical oncology. With 18,000 members representing oncology professionals from over 150 countries worldwide, ESMO is the society of reference for oncology education and information. ESMO is committed to offer the best care to people with cancer, through fostering integrated cancer care, supporting oncologists in their professional development, and advocating for sustainable cancer care worldwide.
191PD_PR - 9 weeks versus 1 year adjuvant trastuzumab for HER2+ early breast cancer: subgroup analysis of the ShortHER trial allows to identify patients for whom a shorter trastuzumab administration may have a favourable risk/benefit ratio
P.F. Conte1, V. Guarneri2, G. Bisagni3, F. Piacentini4, A.A. Brandes5, L. Cavanna6, F. Giotta7, M. Aieta8, V. Gebbia9, A. Frassoldati10, A. Musolino11, O. Garrone12, C. Taverniti13, A. Rimanti14, S. Sarti15, D. Rubino16, A. Bologna17, R. Vicini18, S. Balduzzi18, R. D'Amico18
1Surgery, Oncology and Gastroenterology, University of Padova, Istituto Oncologico Veneto IRCCS, Padua, Italy, 2Department of Surgery, Oncology and Gastroenterology, University of Padova, Istituto Oncologico Veneto IRCCS, Padua, Italy, 3Medical Oncology, Azienda Ospedaliera Arcispedale Santa Maria Nuova - IRCCS, Reggio Emilia, Italy, 4Department of Medical and Surgical Sciences for Children & Adults, Azienda Ospedaliero - Universitaria Policlinico di Modena, Modena, Italy, 5Department of Medical Oncology, Ospedale Bellaria, Bologna, Italy, 6Oncology Department, Azienda Ospedaliera Piacenza, Piacenza, Italy, 7Medical Oncology, Istituto Oncologico Bari, Bari, Italy, 8Medical Oncology, Centro di Riferimento Oncologico Basilicata IRCCS, Rionero in Vulture, Italy, 9Medical Oncology, Ospedale La Maddalena, Palermo, Italy, 10Department of Morphology, Surgery and Experimental Medicine, Azienda Ospedaliera di Ferrara St. Anna, Ferrara, Italy, 11Medical Oncology, Azienda Ospedaliera di Parma, Parma, Italy, 12Medical Oncology, Azienda Ospedaliera St. Croce e Carle, Cuneo, Italy, 13Oncologia Medica Senologica, AOU Città della Salute e della Scienza di Torino, Turin, Italy, 14Oncology, Azienda Ospedaliera di Mantova, Mantova, Italy, 15Medical Oncology, Istituto Tumori della Romagna I.R.S.T., Meldola, Italy, 16Medical Oncology, AOU Policlinico Sant'Orsola Malpighi, Bologna, Italy, 17Medical Oncology, Azienda Ospedaliera Arcispedale Santa Maria Nuova - IRCCS, Reggio Emilia, Italy, 18Department of Diagnostic and Clinical Medicine and Public Health, University Hospital of Modena and Reggio Emilia, Modena, Italy
Background: In the ShortHER trial, 1254 HER2+ early breast cancer patients were randomized to 9 weeks versus 1 year trastuzumab. At 6 years median follow up, non inferiority could not be claimed according to the frequentistic approach (HR 1.13; 90% CI 0.89;1.42) with the upper limit of CI crossing the non inferiority margin set at 1.29. However, based on the pre-planned Bayesian analysis, the probability that the short arm is not inferior to the long one was 80%. Moreover, G >/= 2 cardiac events were 82 in the long and 27 in the short arm (RR 0.33, 95% CI 0.22 to 0.55). It is therefore of interest to identify subgroups of patients for whom, based on the risk/benefit ratio, a shorter treatment might be an option.
Methods: At multivariate analysis, pathologic tumor size and nodal status were independent prognostic parameter for Disease-Free Survival (DFS) and three prognostic groups could be identified: low risk (pT </= 2cm and N0), intermediate risk (pT </= 2cm and any N category or pT > 2cm and N 0-3) and high risk (pT > 2cm and N 4+).
Results: The low, intermediate and high risk groups included 37.5%, 51.9% and 10.5% of the patient population, respectively. The 5 year DFS and G>/=2 cardiac events in the long and short arms according to the prognostic group are summarized below:
Clinical trial identification: EudraCT: 2007-004326-25; NCT00629278
Conclusions: Subgroups analysis of the ShortHER trial allows to identify patients at low and intermediate risk of relapse. This population includes 89% of the patients and the 5 year DFS is not different in the two treatment arms (89% long, 88% short; HR 1.02, 95% CI 0.78-1.33); on the contrary, the risk of cardiac events is significantly higher in the long arm (12.8% vs 4.5%; RR 2.88, 95% CI 1.85-4.47). These results may contribute to better define the risk/benefit ratio of 1 year adjuvant trastuzumab.
Legal entity responsible for the study: University of Padova and University of Modena and Reggio Emilia
Funding: Agenzia Italiana del Farmaco (AIFA).
Disclosure: P.F. Conte: Research funds (Institution): Roche; Speakers' Bureau: Roche/Genentech.
V. Guarneri: Research funds (Institution): Roche.
All other authors have declared no conflicts of interest.
LBA12_PR - PERSEPHONE: 6 versus 12 months (m) of adjuvant trastuzumab in patients (pts) with HER2 positive (+) early breast cancer (EBC): Cost effectiveness analysis results
C. Hulme1, P. Hall2, B. Shinkins1, F. Chehadah1, C. McCabe3, L. Hiller4, J. Dunn4, H.M. Earl5
1Academic Unit of Health Economics, University of Leeds-Institute of Health Sciences, Leeds, UK, 2Edinburgh Cancer Research UK, University of Edinburgh, Edinburgh, UK, 3Faculty of Medicine and Dentistry, University of Alberta, Edmonton, AB, Canada, 4Warwick Clinical Trials Unit, University of Warwick, Warwick, UK, 5Department of Oncology, Cambridge University Hospitals NHS Foundation Trust - Addenbrooke's Hospital, Cambridge, Cambridgeshire, UK
Background: Adjuvant trastuzumab has significantly improved outcomes for HER2+ EBC, using the 12m duration empirically adopted from pivotal registration trials. Given an annual per patient cost of trastuzumab treatment of over £30,000 (Euro35,000), a shorter duration has the potential to improve cost-effectiveness if efficacy is maintained.
Methods: The cost-effectiveness analysis was based on data from the PERSEPHONE trial, a phase III non-inferiority RCT comparing 6 to 12m Trastuzumab, the largest reduced-duration non-inferiority trial internationally. A landmark analysis 6 months into treatment was conducted, comparing costs and quality of life throughout follow-up (6m – 24m post treatment start). Multiple imputation was required to impute incomplete quality of life data. Quality Adjusted Life Years (QALYs) were adjusted for differences at baseline. Uncertainty is estimated using the non-parametric bootstrap method.
Results: 4009 patients were disease free at 6m (6m: n=2000, 12m: n=2009) and therefore eligible for the analysis. Of these, 250 individuals (6m: n=132, 12m: n=118) had either no returned PQ forms or no returned treatment forms and therefore were excluded from the analysis. The average costs for an individual in the 6m arm and 12 month arm were £2,538.64 (95% CI: £2,383.38 - £2700.72) and £12,333.83 (95% CI: £12,098.58 - £12,562.27), respectively, giving an average cost saving of £9,793.25 (95% CI: £9,515.86 - £10,071.64) per individual. Trastuzumab treatment and administration accounted for £9,699.58 (95% CI: £9,436.20 - £9.954.67) of this cost saving, the remaining arising from cardiac assessment and treatment costs and inpatient days. The average QALYs for an individual in the 6m arm and 12 month arm were 0.764 (95% CI: 0.754 – 0.774) and 0.752 (95% CI: 0.742 – 0.763), respectively, giving an average QALY difference of 0.012 (95% CI: -0.002 – 0.026). Thus, the 6m arm dominated with a probability of being cost effective of 94.78%.
Conclusions: 6m of Trastuzumab was shown to be cost effective compared to 12m with cost-savings and no evidence of a detriment to quality of life.
Clinical trial identification: ISRCTN 52968807
Legal entity responsible for the study: University of Leeds
Funding: National Institute of Health Research Health Technology Assessment (NIHR HTA) Programme UK. Funding reference number - 06/303/98.
Disclosure: All authors have declared no conflicts of interest.
This press release contains information provided by the authors of the highlighted abstracts and reflects the content of those abstracts. It does not necessarily reflect the views or opinions of ESMO who cannot be held responsible for the accuracy of the data. Commentators quoted in the press release are required to comply with the ESMO Declaration of Interests policy and the ESMO Code of Conduct.