Growing evidence indicates that anticancer agents can mobilize the immune system against the tumour. By reinstating immunosurveillance, the activity of conventional and targeted therapies might be prolonged beyond cessation of the treatment. Laurence Zitvogel and colleagues explore in an article, published in the Nature Reviews Clinical Oncology, how imatinib operates through immune and cell-autonomous mechanisms with practical implications for defining biomarkers that predict response or resistance to imatinib, as well as how this concept might inform on novel combination regimens that include imatinib with immunotherapies.
Around 15 years ago, imatinib became the very first 'targeted' anticancer drug approved for clinical use. This drug became an example of a successful precision medicine that has truly changed the outcome in patients with Philadelphia-chromosome-positive chronic myeloid leukaemia (CML) and gastrointestinal stromal tumours (GISTs). It targets the oncogenic drivers in these malignancies, BCR–ABL1 and KIT and/or PDGFR.
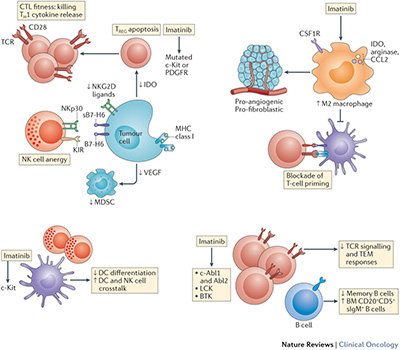
Nonetheless, the aforementioned paradigm might not fully explain the clinical success of this agent in these diseases. Growing evidence indicates that the immune system has a major role both in determining the therapeutic efficacy of imatinib and in restraining the emergence of escape mutations.
Anticancer agents can mobilize the immune system against the tumour by inducing immunogenic death of malignant cells, by sensitizing such cells to an immune attack, by suppressing regulatory immune circuits, or by engaging activating immune receptors. By inducing or reinstating immunosurveillance, the activity of conventional and targeted therapies might be prolonged beyond cessation of the treatment. In the article, the authors explore the case of imatinib to exemplify that targeted anticancer therapies likely operate through 'off-target' or 'immune', in addition to 'on-target' or 'cell-autonomous', mechanisms.
The authors stated a number of arguments that support the notion that imatinib exerts beneficial off-target effects on the immune system that have contributed to its therapeutic success:
- An ample evidence from mouse models indicates that imatinib and other tyrosine kinase inhibitors (TKIs) only mediate long-term anticancer effects in a context in which NK cells and T lymphocytes are fully functional.
- In patients with GIST, the composition and function of the NK-cell and T-cell infiltrates of the tumour predicts clinical outcome after imatinib treatment.
- The functional competence of NK cells (and relative expression of NKp30 isoforms) dictates the long-term outcome of patients with GIST treated with imatinib.
- Imatinib affects the composition of the immune infiltrate of GISTs, and this dynamic parameter affects clinical outcome.
- Imatinib can cause atypical stress and death of CML or GIST cells, and this might contribute to priming and/or recruitment of innate-immune effectors.
- Imatinib directly and indirectly inhibits TREG cells and myeloid-derived suppressor cells, hence removing a major break on anticancer immunity. Some evidence exists that TKIs targeting the same receptor kinases as imatinib can stimulate tumour-specific NK-cell and T-cell responses; however, it remains to be seen whether other TKIs can stimulate anticancer immune responses. For example, BRAF inhibitors might stimulate anti-melanoma immune responses, which might prolong the clinical efficacy of these agents.
Imatinib and next-generation agents have mediated long-term therapeutic effects that, in many patients with CML, persist well beyond cessation of the treatment. It is tempting to speculate that the characteristics of the disease have an important role in generating protective long-term memory T cells.
In patients with GIST, NK cells contribute to short-term and long-term 'adjuvant' effects, but might not coordinate adaptive immune responses given the very rare percentages of complete responders and the dependence upon imatinib administration, with drug cessation leading to immediate relapse. Half of the patients with GIST present with an unfavourable NKp30 isoform phenotype that might account for the failure to elicit antitumour T-cell responses. Specific therapeutic interventions aimed at restoring NKp30 functions are needed.
In addition, imatinib-induced 'immune resistance' involves, at least partly, the dominance of tumour-associated macrophages that should be prevented by interventions that interfere with the CSFR1 signalling pathway or by the use of drugs that kill these cells.
On the basis of these considerations, it will be important to combine imatinib with immunotherapeutic procedures to ameliorate the treatment of GIST and CML. Moreover, the possibility of using imatinib as a general immunostimulator for the treatment of multiple distinct cancers, beyond its usual indication that is restricted to GIST and CML, should be actively explored.
ESMO members: your membership includes access to the article via our journal access page on OncologyPRO.