In a first trial of enzalutamide alone or combined with an aromatase inhibitor in women with advanced breast cancer, pharmacokinetics and tolerability of enzalutamide have shown to be similar to that reported in men with metastatic castration-resistant prostate cancer. Enzalutamide reduced exposure to anastrozole by 88% and to exemestane by 40%. Estradiol remained low in combination with exemestane and 12 of 36 patients enrolled into the aromatase inhibitor cohorts have been on study for 16 weeks or longer, according to the study results presented in a poster session by Dr Tiffany Traina of the Department of Medicine, Memorial Sloan-Kettering Cancer Center, New York, USA at the IMPAKT 2014 Breast Cancer Conference (8-10 May 2014, Brussels, Belgium).
Androgen receptor positive breast cancer
Enzalutamide is a potent novel oral inhibitor of the androgen receptor which is expressed in a majority of breast cancers. It demonstrated preclinical activity in all breast cancer subtypes that express the androgen receptor and thus could be effective in androgen receptor-positive breast cancer, the authors explained in background of the study.
Androgens are converted by an enzyme, aromatase, to estrone and estradiol. Aromatase inhibitors block the conversion of androgens to estrogens by inhibiting aromatase, resulting in a concomitant increase in androgens. Enzalutamide may add to the activity of aromatase inhibitors by blocking potential growth stimulation of the androgen receptor due to increased circulating androgens. Enzalutamide is a potent CYP3A4 inducer. Both exemestane and anastrazole are metabolised by CYP3A4. Stage 2 of this trial investigated whether any observed drug-drug interaction between enzalutamide and these aromatase inhibitors would translate into an effect on circulating estrogens.
Stage 1 of this phase I study in patients with advanced breast cancer evaluated single agent enzalutamide at daily doses of either 80 or 160 mg. Blood was collected for pharmacokinetic analysis. Dose limiting toxicities were recorded through day 35.
Stage 2 of the trial evaluated daily enzalutamide at 160 mg dose and added to patients receiving either anastrozole 1 mg or exemestane 25 mg. In stage 2, blood for pharmacokinetics and hormones was collected pre- and post-enzalutamide treatment.
Tumour assessments were performed approximately every 3 months in both stages.
Stage 1 of the study with 15 included patients confirmed 160 mg of enzalutamide as the daily dose for further study in women with advanced breast cancer. A single dose limiting toxicity, in particular adrenal insufficiency, occurred at dose of 80 mg.
As of October 2013, 20 patients were enrolled to anastrozole and 16 to exemestane. Median age was 57 years, performance status 1 according ECOG scale, and patients received 5 prior therapies for advanced disease in stage 1 study. However, in stage 2 median age was 59 years, performance status 0 and the patients received 4 prior lines of therapy for their cancer, respectively.
In stage 1 of the study, common (>15%) treatment-related adverse events of any grade included AST elevation, nausea, and nasal congestion. In stage 2 common treatment-related adverse events of any grade were fatigue, anorexia, nausea, hot flush, and vomiting. Grade ≥3 adverse events in at least 2 patients were anaemia in stage 1 and fatigue in stage 2.
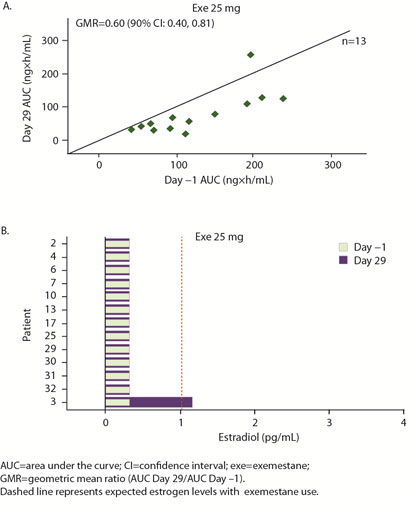
The geometric mean ratio of day 29/day 1 AUC for anastrozole was 0.12 and for exemestane 0.60, meaning that enzalutamide reduced exposure to anastrozole by 88% and to exemestane by 40%.
Preliminary hormone data showed increased estradiol on day 29 over day 1 in 7 of 14 patients in anastrozole group vs. 1 of 12 in exemestane group. The authors concluded that estradiol remained low in combination with exemestane, but possibly not with anastrozole. Exemestane with or without enzalutamide is being evaluated in a randomized phase II study.
Exemestane with or without enzalutamide is being evaluated in randomised phase II studies. Three global phase II clinical trials are enrolling:
- MDV3100-11: single agent enzalutamide in androgen receptor positive triple negative breast cancer with primary endpoint of clinical benefit rate.
- MDV3100-12: a randomised trial investigating exemestane plus enzalutamide versus exemestane plus placebo in hormone receptor positive breast cancer with a primary endpoint of progression-free survival.
- 9785-CL-1121: an open label study investigating enzalutamide with trastuzumab in HER2-positive, androgen receptor positive metastatic or locally-advanced breast cancer with a primary endpoint of clinical benefit rate of at least 24 weeks
Reference
Abstract 10P: A Phase 1 open-label study evaluating the safety, tolerability, and Pharmacokinetics of enzalutamide alone or combined with an aromatase inhibitor in women with advanced breast cancer
Annals of Oncology (2014) 25 (suppl_1): i4-i4. 10.1093/annonc/mdu064
Dr Traina reported that serves as advisory board member for Genentech, Eisai and Prostrakan. She received honoraria from Genentech, Celgene, Merck, Eisai and Prostrakan. She received research funding from Medivation, AstraZeneca, Eisai, Ziopharm, Janssen, Genentech and Novartis. Other authors disclosed that Dr Yardley provides consultancy and is advisory board member for Medivation; Dr Patel receives honoraria for speakers bureau from Medivation; Dr Blaney is an employee of Medivation; Dr Gibbons is an employee of Medivation; and Dr LoRusso receives research grant and provides consultancy for Astellas. All other authors have declared no conflicts of interest.
IMPAKT is an annual conference that was launched in 2009 by the Breast International Group (BIG) and the European Society for Medical Oncology (ESMO), in collaboration with a multidisciplinary alliance of European breast cancer organisations and patient groups - referred to as partners. Partners include the Foundation St. Gallen Oncology Conferences and the EBC Council. It is designed for breast cancer researchers and clinicians who have a specific interest in translational research, new agents, molecular and functional diagnostics, biomarkers and cutting-edge research applications in the clinical setting. The theme of IMPAKT 2014 was Anticipating the future of personalised medicine in breast cancer.