Regional hyperthermia as induction added to neoadjuvant chemotherapy enhanced clinical benefit across all measured parameters in patients with localised high-risk soft tissue sarcoma according to phase III study results reported at the European Cancer Congress (ECC 2015), convening in Vienna, Austria, 25 - 29 September, 2015.
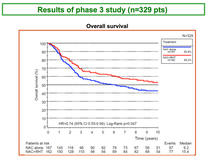
Overall survival (OS) results from a long-term follow-up of the randomised, multicentre, phase III EORTC trial (EORTC 62961/ESHO; registered: NCT00003052) of neoadjuvant chemotherapy alone or combined with regional hyperthermia in patients with localised high-risk soft tissue sarcoma, showed a median OS of 15.4 years for patients receiving neoadjuvant chemotherapy plus regional hyperthermia compared to 6.2 years for patients receiving only neoadjuvant chemotherapy.
Previously reported findings (Issels et al. Lancet Oncol 2010) demonstrated that the addition of regional hyperthermia to neoadjuvant chemotherapy significantly improved local progression-free survival (LPFS) and disease-free survival (DFS) in patients with localised high-risk, soft tissue sarcoma, according to Rolf Issels, Klinikum der Univers. München-Großhadern Klinikum Grosshadern, Medical Clinic III, Munich, Germany.
Prof. Issels reported findings at the Proffered Paper: Sarcoma: Soft Tissue and Bone session from an analysis of a long-term follow-up that evaluated whether the reported improvements are sustained and translate into an OS benefit.
Long-term study results show significantly prolonged OS rates
This analysis was done on data from 167 patients treated with neoadjuvant chemotherapy and 162 patients treated with neoadjuvant chemotherapy plus regional hyperthermia comprising the intent to treat population. All patients had localised high-risk soft tissue sarcoma of 5 cm or larger that were FNCLCC grade 2 or 3.
Patients were stratified by site, disease presentation, and centre and randomised to receive either etoposide 125 mg/m², ifosfamide 1500 mg/m² plus adriamycin at 50 mg/m² for 4 cycles either alone or together with regional hyperthermia at 42°C for 60 min on days 1 and 4 as induction therapy. Following local therapy, another 4 cycles of allocated treatment was given as adjuvant therapy. Baseline and disease characteristics, including concomitant local interventions of surgery and/or radiotherapy, were well-balanced between study arms.
The primary endpoint of the study was LPFS and secondary endpoints included treatment safety, response, DFS, and OS.
The median follow-up was 74 months in 329 patients (155 censored: 132 months).
By December 2014, 221 (67%) [95% CI 62%, 72%]) patients had relapsed and 174 (53% [95% CI 47%, 58%]) had died.
Significantly prolonged OS was observed in patients receiving regional hyperthermia compared with patients receiving only chemotherapy alone; hazard ratio [HR] 0.74; 95% CI 0.55, 0.99 (log rank p = 0.047).
At the 5-year follow-up, OS in patients receiving regional hyperthermia was 63% (95% CI 55%, 70%) compared to 51% (95% CI 43%, 59%) in the chemotherapy arm.
LPFS rates were 51% versus 40% and DFS was 42% versus 34%, HR 0.72; 95% CI 0.55, 0.94 (log rank p = 0.016).
After 9 years of follow-up, OS rates of 54% (95% CI 46%, 62%) compared to 43% (95% CI 35%, 50%) were demonstrated in the hyperthermia arm compared to the adjuvant chemotherapy arm, respectively. LPFS also was also significantly higher in the hyperthermia arm compared to the adjuvant chemotherapy arm; HR 0.71; 95% CI 0.54, 0.93 (log rank p = 0.012).
The toxicity profile was consistent with prior experience of neoadjuvant chemotherapy and hyperthermia; no unexpected or new safety findings were reported.
Prof. Jean-Yves Blay, Director General of the Centre Leon Berard, Université Claude Bernard Lyon 1, Lyon, France, who discussed the study results said that it is a positive randomised trial for primary endpoint and overall survival. It was performed in high-risk population and contributed to cure in significant number of patients. However, comparator arm is not a universal standard. Technology used in the study should be disseminated. Confirmatory trial(s) is(are) warranted. The reimbursement is also ope issue.
Conclusions
These findings strongly support adding regional hyperthermia to standard neoadjuvant chemotherapy in patients with localised high-risk soft tissue sarcoma. Induction with hyperthermia resulted in significantly increased OS, DFS, and LPFS.
The authors suggest that the beneficial effects on survival might be linked to the known heat shock related immune effects induced by hyperthermia.
Reference
13LBA Improved overall survival by adding regional hyperthermia to neoadjuvant chemotherapy in patients with localized high-risk soft tissue sarcoma (HR-STS): Long-term outcomes of the EORTC 62961/ESHO randomized phase III study.